Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on October 12th, 2023  Naked mole rat (Meghan Murphy, Smithsonian’s National Zoo, Flickr. https://flic.kr/p/awZo8C) 12 Oct. 2023. An organization funding research on extending the human life span by preventing age-related diseases is founding its first biotechnology company. VitaDAO, a group that calls itself “a community-owned collective” raising funds through decentralized blockchain-based finance, announced the start-up and seed funds for Matrix Biosciences, a company advancing longevity research from labs at University of Rochester in New York. (DAO stands for decentralized autonomous organization.)
VitaDAO is a two year-old enterprise that says it funds early-stage research on longevity, by better understanding, diagnosis, and control of age-related diseases to extend the human life span. The group says it seeks to commercialize promising translational research on aging from academic labs, particularly higher-risk initiatives but also with high reward potential. VitaDAO says it so far has committed $4 million to support 20 projects in longevity research, with major decisions made through the consent of its funders.
While VitaDAO’s official base is Berlin, it operates as a decentralized entity, and in June 2023 raised $4.1 million in seed funds from drug maker Pfizer, Shine Capital, and technology angel investor Balaji Srinivasan, among others. For ongoing finance, the organization says it uses non-fungible tokens or NFTs, an application of blockchain technology to identify and define unique items of value, in this case the underlying intellectual property for research discoveries from VitaDAO’s funded projects, called IP-NFTs. VitaDAO members can also purchase or earn tokens through donations of intellectual property that give holders voting privileges on the group’s direction and initiatives.
Abundance of high molecular weight hyaluronic acid
While VitaDAO has up to now backed research projects, Matrix Biosciences represents the organization’s first spin-off biotechnology company. Matrix Bio is commercializing research from the lab of biology professor Vera Gorbunova at University of Rochester, also co-director of the Rochester Aging Research Center. Gorbunova studies the genomes of long-living mammals, seeking mechanisms for genome stability and longevity that can be applied to humans. One of those species studied by Gorbunova is the naked mole rat that lives up to 40 years, while most other rats have a life span of about three years.
One of the contributing factors to this longer lifetime is the naked mole rat’s abundance of high molecular weight hyaluronic acid that is believed to give that species a higher resistance to cancer. Matrix Bio expects to conduct preclinical studies of hyaluronic acid compounds to discover an optimal candidate for treating cancer and other age-related diseases. VitaDAO says Matrix Bio also plans to develop a class of small molecule or low molecular weight drugs that control enzymes for breaking down hyaluronic acid as candidates for treating cancer other age-related disorders. The group is providing seed funds of $300,000 and plans to supplement that amount in early 2024 with funds raised through IP-NFT tokens.
“Matrix Biosciences,” says Anthony Schwartz, a biotechnology executive tapped to lead Matrix Bio in a VitaDAO blog post, “is navigating a complex development pathway commonly encountered by many early-stage companies during the initial stages of drug discovery.” Schwartz adds, “We are pleased with the early data generated thus far and its potential for advancing into clinical trials.”
More from Science & Enterprise:
Disclaimer: The author owns shares in Pfizer.
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on October 11th, 2023  (Darryl Leja, National Human Genome Research Institute, NIH. Flickr: https://www.flickr.com/photos/genomegov/30395469313) 11 Oct. 2023. A developer of therapies addressing the body’s microbe communities is selling off key assets to another biotechnology company, after problems developed with its lead program. Kanvas Biosciences in Princeton, New Jersey is acquiring two of Federation Bio’s microbiome therapy candidates, as well as Federation’s microbial library and other intellectual property.
Federation Bio was a biotechnology company in South San Francisco developing treatments for disorders linked to the microbiome, communities of bacteria in the gut and elsewhere. The microbiome is generating more interest as a target for diseases of the gut, but also conditions in other parts of the body. The company, whose web site is no longer active, said it discovers and isolates collections of naturally occurring bacterial strains for long-term treatments that supplement existing microbes in the body. Federation Bio was based on research by its scientific founders Michael Fishback, professor of biomedical engineering and microbiology, and Dylan Dodd, professor of pathology, both at Stanford University.
Science & Enterprise reported on the company’s first venture round in Oct. 2020 raising $50 million, and most recently a collaboration between the company and M.D. Anderson Cancer Center in Houston in Feb. 2023, studying links between the gut microbiome and effectiveness of cancer immunotherapies, particularly immune checkpoint inhibitors. The company’s lead product, code-named FB-001, was a treatment for hyperoxaluria, a kidney disorder from excessive oxalate in urine that can lead to kidney stones and damage to the kidney. Federation Bio said FB-001 was an oral drug made with 148 bacterial strains from healthy donors.
In Dec. 2022, Federation Bio announced the start of an early-stage clinical trial among healthy volunteers, but by June 2023, problems began developing in the company that required layoffs, as reported by Fierce Biotech. Today, Kanvas Biosciences says it’s acquiring several of Federation Bio’s current programs, facilities, and intellectual property. Financial terms of the agreement are not disclosed.
Mapping and imaging of microbial communities
Kanvas Biosciences is a three year-old biotechnology company with a process for detailed mapping and imaging of microbial communities. This spatial mapping technology, as described in the journal Nature in Dec. 2020, analyzes single-cell images to reveal locations and identities of bacteria and other microbes in the gut, aided by machine-learning algorithms. Those techniques, studied in the biomedical engineering lab of Iwijn De Vlaminck at Cornell University, are the basis of Kanvas Biosciences’ process. Hao Shi and Philip Burnham, chief technology officer and chief scientist respectively of Kanvas Biosciences, are alumni of that Cornell lab.
In their agreement, Kanvas Biosciences is acquiring Federated Bio’s work in cancer immunotherapy, and inheriting the company’s collaboration with M.D. Anderson Cancer Center begun in February. That collaboration is expected to lead to development and manufacturing of fecal transplants with synthetic microbes to help more cancer patients become responsive to immunotherapies. Kanvas is also gaining work begun by Federated Bio on treatments for inflammatory bowel disease. In addition, Kanvas receives Federation Bio’s microbial cell libraries and banking facilities in South San Francisco, and other intellectual property assets.
“This acquisition,” says Kanvas Biosciences CEO Matthew Cheng in a company statement released through BusinessWire, “allows us to manufacture and clinically investigate complex microbial consortia much faster than previously envisioned.” Cheng co-founded Kanvas Biosciences with Hao Shi in early 2021.
“Our work with Federation Bio demonstrated our unique ability to engineer complex, synthetic microbial consortia,” notes Jennifer Wargo, professor of genomic medicine and surgical oncology at M.D. Anderson. Wargo adds, “This agreement allows us to accelerate an already productive collaboration, and we hope it will enable us to more quickly develop and manufacture more microbiome-based therapies for our patients.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on October 10th, 2023  (Rawpixel, Pixabay) 10 Oct. 2023. A group of managed care organizations is examining the value of real world data from Europe and the U.S. to help FDA review alternative biologic drugs. The Food and Drug Administration is giving the Biologics and Biosimilars Collective Intelligence Consortium or BBCIC a grant of $1.4 million to assess if real world data from outside the U.S. can play a role in reviewing new so-called biosimilar therapies.
Biosimilars are biologic therapies derived from live cells or microorganisms, such as yeast or bacteria, for treating some chronic and immune-related diseases, which are given to patients and work in the body the same way as the original branded biologics. Unlike generic drugs made as copies of the original brand-name medications, biosimilars may have minor variations from the original biologic chemistry, but they need to show the same purity, potency, and safety of the original product. And like generics, biosimilars are offered as less expensive treatment options as original branded biologics.
BBCIC in Alexandria, Virginia is a not-for-profit organization that evaluates real world safety and effectiveness of biologic drugs, including biosimilars. The group was formed by the Academy of Managed Care Pharmacy in 2015 and includes managed care organizations, research institutes, pharmaceutical companies, and pharmacy benefit managers that administer prescription drug programs and negotiate discounts for health insurance plans. BBCIC conducts research on use of biologics and biosimilars, including real-world experiences and outcomes from using these medications in clinical settings, after their approval by regulatory agencies.
Value of overseas data for regulatory review
The study for FDA seeks to assess the value of real world data, such as de-identified medical records and insurance claims from outside the U.S., to that agency’s regulatory review of biosimilar candidates and other actions. The study team is looking specifically at real world data from Italy and Denmark, as well as the U.S. Leading the research are Gianluca Trifirò, professor of pharmacology at University of Verona in Italy and Jesper Hallas, professor of clinical pharmacology at University of Southern Denmark in Odense.
Cate Lockhart, BBCIC’s executive director, says in an Academy of Managed Care Pharmacy statement that findings from the study can help speed FDA’s review of biosimilars, with benefits for patients. “The current approval process for biosimilar products is complex,” says Lockhart, “delaying patients’ access to cost-effective therapies. Using real world data from foreign countries will help improve the FDA’s work and ultimately help patients receive access to approved therapies in a timelier manner.” Lockhart adds that the study “aligns with the FDA’s mission to make safe and effective drugs available to patients nationwide.”
Real world data from electronic health records and insurance claim databases are becoming more valuable resources to guide medical decision-making, to supplement or follow-up on clinical trials, as well as for regulatory agencies. In April 2023, Science & Enterprise reported on a medical journal article with an analysis of insurance claims records matching the design of randomized clinical trials, which shows claims data can in some cases resemble clinical trial results to make causal inferences. The paper draws on work by the RCT-Duplicate project — RCT standards for randomized clinical trials — that receives financial support from FDA.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on October 9th, 2023  (Gerd Altmann, Pixabay) 9 Oct. 2023. A developer of treatments for Alzheimer’s disease that seeks to improve the condition of the brain’s support cells is raising £48 million ($US 61 million) in its first venture round. AstronauTx Ltd. is a four year-old company in London, started by the Dementia Discovery Fund to advance therapies with alternative mechanisms of action from other techniques showing limited results.
The company says it focuses on a type of cell called astrocytes to improve the brain’s ability to clear toxic accumulations such as amyloid-beta and tau associated with Alzheimer’s disease and other dementias. Astrocytes are a subset of glial cells that make up most of the cells in the brain and support the functioning of neurons, cells that send and receive electrical signals in the brain and elsewhere. Among astrocytes’ key tasks are protecting the central nervous system by clearing excess neurotransmitters and other potentially damaging substances.
AstronauTx says it’s developing treatments that boost the ability of astrocytes to perform their protective roles, while enhancing the cells’ metabolic functions and emission of hormones for the long-term health of neurons. In people with Alzheimer’s disease, says the company, these functions are overwhelmed as neurodegeneration takes hold and progresses. At its founding, AstronauTx said it planned to build on research at the Alzheimer’s Research UK Drug Discovery Alliance at University College London.
Rare variant associated with degenerative effects
While AstronauTx does not reveal precise details of its technology, the work of the company’s chief scientist Jamie Bilsland offers a few hints. In 2019, Bilsland co-authored a paper identifying a rare variant in the phospholipase C-gamma 2 or PLCG2 gene that codes for a signaling enzyme in immune cells, which the authors associate with late-onset Alzheimer’s disease. A more recent paper highlights further associations of this variant enzyme with degenerative effects on glial cells in the brain, such as astrocytes.
Also, in July 2023, AstronauTx agreed to a licensing deal with the Danish biotechnology company Saniona that discovers new therapies targeting ion channels on cell membranes forming tiny pores in cells to encourage cell signaling. Dysfunctions in ion channels on astrocytes are associated with several neurodegenerative disorders, including Alzheimer’s disease. The agreement calls for the two companies to collaborate on discovering neurodegenerative disease treatments affecting an undisclosed ion channel target.
AstronauTx is raising £48 million in its first venture funding round, led by the Novartis Venture Fund, the corporate venture arm of drug maker Novartis that backs biotechnology and biopharmaceutical start-ups in Europe, North America, and Israel. Joining the round are MPM Capital, Brandon Capital, EQT Life Sciences, Bristol Myers Squibb, and the company’s founding investor Dementia Discovery Fund.
Proceeds from the venture round are expected to advance the company’s lead candidate for Alzheimer’s disease, but also other neurodegenerative disorders. “Our treatments will be oral drugs,” says AstronauTx co-founder and CEO Ruth McKernan in a company statement, “applicable across multiple neurodegenerative conditions, and additive with mechanisms that are currently in late-stage development.” McKernan adds, “We now know that the processes causing Alzheimer’s and other similar diseases are modifiable. Progress towards a compendium of new drugs against these devastating diseases is thankfully well underway.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on October 7th, 2023 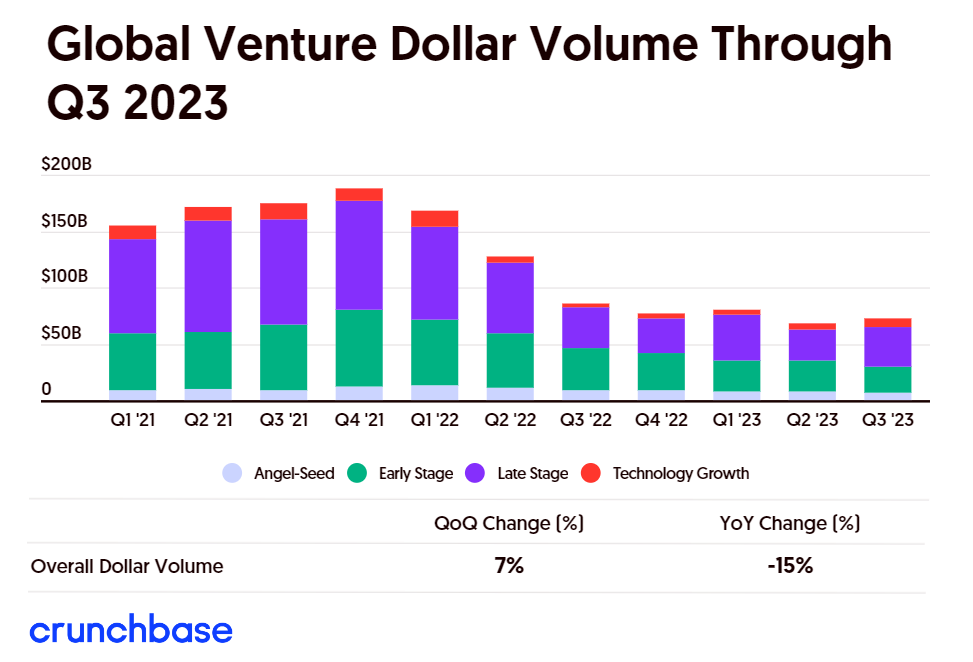 Click on image for full-size view. (Crunchbase) 7 Oct. 2023. Global venture investment dollars in the third quarter of 2023 rose somewhat from the previous quarter, but remained at about the same level for the past 12 months. The technology market research company Crunchbase reported on Thursday that total venture investments worldwide rose to $73 billion from July through September 2023, a seven percent gain from the second quarter, but still down 15 percent from the same period in 2022.
Crunchbase says much of the investment dollar increase went to late-stage funding — in companies’ third venture rounds, called series C, or later. Late-stage investments worldwide totaled $43 billion or 59 percent of the total for the third quarter, a gain of 30 percent from the second quarter and 10 percent over the the third quarter of 2022. But even with that big quarterly jump in total dollars, the number of late-stage investment deals dropped slightly from 504 in Q2 to 490 in Q3. Global early-stage investments — first and second venture rounds — and seed-stage dollars and deals both declined from the second quarter.
Artificial intelligence start-ups continued to attract many of the investments, according to Crunchbase, collecting about $10 billion of the $73 billion total. Semiconductor and sustainability technology enterprises also attracted many of the investment dollars, with nearly all (96%) of the $4.5 billion in semiconductor investments made in Chinese companies.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on October 6th, 2023  (A. Kotok, Flickr. https://flic.kr/p/7k4ebZ) 6 Oct. 2023. A biotechnology company is receiving NIH support for discovering antibodies that address the vast majority of proteins coded by the human genome, passed-up so far by drug makers. Integral Molecular in Philadelphia is receiving nearly $900,00 from National Institute of General Medical Sciences or NIGMS, part of National Institutes of Health, for the two-year project.
Integral Molecular discovers and designs monoclonal antibodies, synthetic proteins with immune system properties, as therapies for disease-causing proteins residing on cell membranes, particularly targets considered difficult to reach by conventional means. The company says it specializes in targeting suspected membrane proteins with complex chemistries or well hidden binding sites, called epitopes, and those with rare properties. Integral Molecular offers its antibody discovery technology as a service, and has a pipeline of several antibody candidates in preclinical stages.
The company says it produces monoclonal antibodies by starting with antigen proteins designed to invoke specific responses in protein chemistries. Integral Molecular says it employs virus-like particles and messenger RNA or mRNA in its antigens that create native-like immune responses from cell-membrane proteins. To generate new antibodies, the company says it uses genetically diverse yet humanized engineered chickens as host organisms, whose cells produce varied, but humanized antibody protein responses. Integral Molecular says engineered antibodies from genetically modified chickens are compatible with lab animals for preclinical study as well as humans.
90 percent of proteins not targeted
The award supports NIH’s Illuminating the Druggable Genome initiative, research across multiple NIH institutes and centers to gain a better understanding of the estimated 90 percent of some 4,000 human proteins not targeted by approved drugs. The project focuses on a few collections of these difficult proteins, including G-protein-coupled receptors or GPCRs and ion channels. GPCRs are a large and diverse set of receptor proteins found on cell membranes that play a variety of key functions in the body, with as many as half of all current drugs binding to GPCRs. Ion channel receptors are also evident on some cell membranes, but perform more specialized functions, allowing ion channel pores in the membranes to open or close in response to chemical or mechanical signals. Ion channels are found on excitable cells, such as neurons and in muscles and sensory cells.
To support this initiative, Integral Molecular is discovering engineered monoclonal antibodies addressing 230 GPCRs and ion channels. In a company statement, Ross Chambers, the company’s vice-president for antibody discovery and lead investigator on the project says, with Integral Molecular’s “experience studying complex proteins and an antibody success rate exceeding 95 percent, we are confident in delivering the desired molecules.” Chambers adds, “Our isolation of antibodies reactive with diverse species will help reduce the use of non-human primates in research.”
The award is a Small Business Innovation Research or SBIR grant made under NIH’s small business programs that set aside a part of the agency’s research funding for U.S.-based and owned companies. Most SBIR grants are made in two parts: a first phase to determine technical and commercial feasibility, and a second phase to develop and test a working prototype or prepare for clinical trials. NIH says it allocates $1.3 billion for its small business programs.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on October 5th, 2023 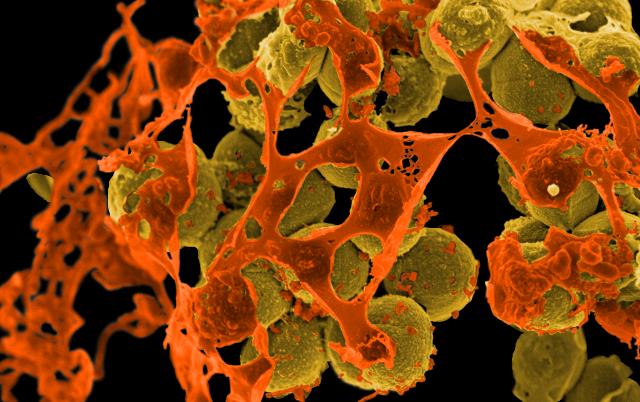 Scanning electron micrograph of methicillin-resistant Staphylococcus aureus, or MRSA, in brown spheres, surrounded by cellular debris. (NIAID, NIH) 5 Oct. 2023. Research with lab mice shows an experimental vaccine stimulates the body’s basic immune system to protect against a range of pathogens associated with hospital infections. A team from University of Southern California, Los Angeles General Medical Center, and a spin-off company ExBaq LLC published their findings in yesterday’s issue of the journal Science Translational Medicine (paid registration required).
According to Centers for Disease Control and Prevention, as of 2018, one in 31 hospital patients will acquire at least one infection as a result of a hospital stay. Since that time, due in large part to more extended hospital visits from the Covid-19 pandemic, some types of health care-acquired infections increased, particularly ventilator-associated infections. During that same time, Clostridioides difficile or C. diff infections decreased, attributed to better hand-washing practices. Some of the continuing so-called superbugs, such as methicillin-resistant Staphylococcus aureus or MRSA, spread on contaminated surfaces, such as catheters, with patients in intensive care units often at higher risk.
The study team is seeking better tools for health care facilities to fight-off infections in their most vulnerable patients. Even with the infection problem increasing, vaccines to prevent infections are designed to address single pathogens, not the collection of bacteria and fungi that can infect hospitalized patients. As a result, the researchers use a strategy to stimulate the innate immune system, the body’s fact-acting first line of defense against infections that responds to suspected invaders.
To invoke the innate immune system, the team designed a vaccine to stimulate a response from macrophages, white blood cells in the immune system that first appear as cells called monocytes, precursors to macrophages. Monocytes enter the infected areas then transform into large macrophage cells to fight-off the invading pathogen. In addition, when macrophages digest target pathogens, they express antigens on their surface that attract more infection-fighting cells.
No proteins in the vaccine
“This is very different from developing new antibiotics,” says USC doctoral candidate and lead author Jan Yun in a university statement. “This is using our own immune system to fight against different superbugs, which is a different approach than everybody else.”
To generate a macrophage response, the researchers designed a vaccine with aluminum hydroxide, monophosphoryl lipid A, and mannan. Aluminum hydroxide is an adjuvant that enhances immunity, while monophosphoryl lipid A stimulates the transcription factor NF-KB, a key regulator of innate and adaptive immunity. Both aluminum hydroxide and monophosphoryl lipid A are found in vaccines already approved by FDA. Mannan is a naturally occurring fungal compound that can also stimulate monocyte and macrophage responses. None of the ingredients are proteins.
The researchers tested the vaccine on lab mice induced with blood or lung infections from several leading hospital-acquired bacteria: MRSA, vancomycin-resistant Enterococcus faecalis, Escherichia coli or E. coli, and resistant strains of Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Mice given the vaccine, says the team, show reduced bacterial burden and longer survival times. In addition, when given as preventive measure, the vaccine protects lab mice against infections from the fungi Rhizopus delemar and Candida albicans. The researchers report the vaccine takes effect in mice within 24 hours and a single dose protects for up to 28 days.
Yan, with co-authors Travis Nielsen, Brian Luna, and Brad Spelberg founded the company ExBaq LLC in 2017 to further develop and commercialize their discoveries. The company licenses its technology from USC, and in 2020 received a Small Business Technology Transfer award from National Institute of Allergy and Infectious Diseases, part of NIH, to advance the vaccine through pre-clinical stages, with Spelberg — chief medical officer at L.A. General — as lead investigator.
In an ExBaq statement released through Globe NewsWire, Spelberg says the next task is to prepare for FDA clinical trial clearance. “The next step is getting guidance from the FDA on the requirements to complete preclinical studies and submit an Investigational New Drug Application in 2024,” says Spelberg. “The first such trial would be done in healthy volunteers to find the right dose of vaccine that is safe and triggers the same kind of immune response in people as seen in the mice.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on October 4th, 2023 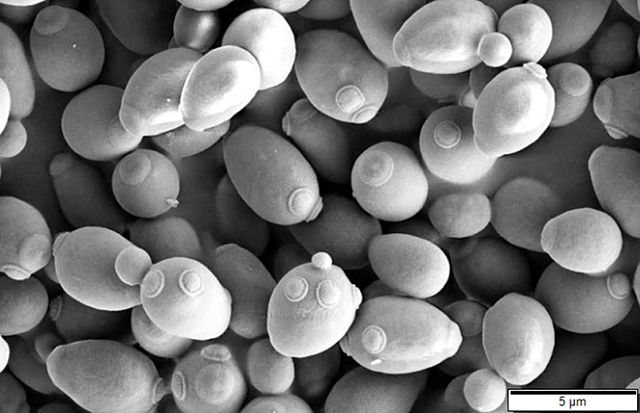 Scanning electron microscope image of Saccharomyces cerevisiae, or baker’s yeast (Mogana Das Murtey and Patchamuthu Ramasamy, Wikimedia Commons) 4 Oct. 2023. A company offering a biomanufacturing process it says is more efficient and scalable than conventional bio-production methods is raising $9.5 million in its first venture round. Pow.Bio in Berkeley, California is a four year-old enterprise employing techniques for continuous rather than batch production of synthetic biomaterials, such as bio-based chemicals and plastics, and building a demonstration facility for its process in nearby Alameda, California.
Pow.Bio seeks to provide developers of bio-based products across a range of industries with a continuous fermentation process that enables production in smaller quantities and lower costs than current batch production methods. Today’s biomanufacturing techniques, says Pow.Bio, requires increasing the size and thus the expense of batch-style reactors to remain scalable, raising production costs. Achieving continuous fermentation of engineered microorganisms such as yeast or bacteria, however, has been hampered up to now by contamination and genetic changes called drift among biomass cells in reactors, making this alternative technique unreliable.
The company says its process succeeds by breaking down biomanufacturing into modular functions that make continuous fermentation possible at scale. Ouwei Wang, a microbiologist trained at University of California in Berkeley and Pow.Bio co-founder, says in a company statement released through BusinessWire, “By running a fermentation process more like an assembly line, we see multi-fold increases in productivity without contamination or drift.” Wang, now Pow.Bio’s chief technology officer, is lead author of a 2017 paper on biotechnology applications of microbial interactions with chemicals, while a doctoral candidate at UC-Berkeley.
Wang founded the company, originally named POW Genetic Solutions, with biotech executive Shannon Hall in 2019. Under that name, the company received a Small Business Innovation Research grant from National Science Foundation in 2019 to address the issue of contamination that limits advances in continuous fermentation. Wang, the lead investigator on that project, proposed a natural biocide to attack microbial contaminants, and to introduce a biocide-resistant enzyme for protecting host fermentation organisms.
Optimize the process and allow for autonomous operations
Pow.Bio says its process has advanced to building a facility that demonstrates the technology end-to-end under real-world conditions. The company says the Alameda facility will also exhibit its management software in action that uses algorithms to optimize the process and allow for autonomous operations. With that software, Pow.Bio says its demonstration facility can show the scaling up of production from gram-scale experiments to full-scale outputs measured in hundreds of kilograms.
And the company claims the process demonstrated in that facility can reduce commercial-scale biomanufacturing expenses by 40 to 70 percent, through lower unit fermentation costs. Pow.Bio contends building more batch production capacity alone cannot match that performance. “Focusing on building capacity,” says Hall, now the company’s CEO, “without addressing unit economics will expose the industry to more frustration about not delivering on its promises. “The right target is economic viability, and hitting it requires technical advances in biomanufacturing.”
Pow.Bio is raising $9.5 million in its first venture funding round led by Hitachi Ventures in Munich, Germany, the venture capital arm of technology products maker Hitachi Ltd. Taking part in the round are Possible Ventures, XFactor Ventures, iSelect, Climate Capital, Vectors, Better Ventures, and Cantos.
Hideshi Nakatsu, CEO of Hitachi’s water and environment business unit, notes, “Pow.Bio’s continuous fermentation platform represents a potential step-change in biomanufacturing economics that aligns with Hitachi’s expansion plans to enter into the bio-production business.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on October 3rd, 2023  (Gerd Altmann, Pixabay) 3 Oct. 2023. An alliance of academic labs and venture investors is starting a new research enterprise to speed development of treatments for rare diseases in the U.S. and U.K. The Oxford-Harrington Rare Disease Centre, located in Oxford, U.K. and Cleveland, Ohio, says its Therapeutics Accelerator plans to commit £200 million ($US 241.3 million) to deliver 40 new therapies over the next 10 years.
The Oxford-Harrington Rare Disease Centre is a four year-old collaboration between University of Oxford and Harrington Discovery Institute, a translational research accelerator affiliated with University Hospitals in Cleveland. The Rare Disease Centre seeks to combine research expertise at Oxford’s labs and Harrington’s new-drug development efforts to support new technologies that address unmet needs from rare diseases. The center says about 400 million people worldwide are living with some 700 rare diseases rare diseases, about half of whom are children. Most rare diseases, says the center, are genetic in origin, but only about five percent of rare diseases have treatments approved by regulatory agencies in the U.S. and Europe.
The Therapeutics Accelerator is a program to identify and advance new innovative treatments for rare cancers as well as neurological diseases and developmental disorders from academic labs in the U.S. and U.K. The program expects to operate under a hybrid non-profit and for-profit model that combines research grants with private investment to develop and bring new therapies to market, including the launch of new spin-off companies.
First investment made last month
University Hospitals and Oxford Science Enterprises, the university’s venture capital arm, are providing financial support for the accelerator under their current co-funding and co-investment agreement. The partners are also establishing a separate Rare Disease Impact Fund to make possible further investments in rare disease therapies.
The Therapeutics Accelerator made its first investment in September to AlveoGene, a start-up company in Oxford advancing gene therapies delivered by inhalation through the lungs to treat rare inherited respiratory diseases. AlveoGene is spun-off from the U.K. Respiratory Gene Therapy Consortium, a group of researchers studying inherited respiratory diseases at universities of Oxford and Edinburgh, and Imperial College London. Science & Enterprise reported last month on the AlveoGene launch and seed funding.
Matthew Wood, a neuroscience professor at Oxford and director of the Oxford-Harrington Rare Disease Center or OHC says in a statement, “OHC was set up with a bold vision and ambitious goals to make an impact on the treatment of rare diseases. This new Accelerator initiative takes this a step further with its unique collaboration and for-profit and not-for-profit model. It is only by combining resources and adopting innovative approaches that we can truly accelerate the development of new drugs for patients in need.”
David Cameron, the former U.K. Prime Minister, chairs the the Oxford-Harrington Rare Disease Center advisory board. Cameron notes, “I know from my own experience of having a son born with an incredibly rare and incurable disease, how little we really know about rare diseases.” He adds, “This Accelerator will deliver new, real and tangible therapies for patients and, by doing so, offer huge benefits for patients, their families and wider society.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on October 2nd, 2023  (DennisM2, Flickr. https://flic.kr/p/SjHYyM) 2 Oct. 2023. The Food and Drug Administration authorized marketing of a genetic test for detecting hundreds of gene variants associated with a higher risk of developing certain cancers. The agency announced its authorization on Friday of the Common Hereditary Cancers Panel, a test provided by Invitae Corp. in San Francisco.
The Common Hereditary Cancers Panel assesses blood or other sample specimens for variations in 47 genes in the human genome. The specimen sample is taken by a clinician, then sent to a lab for high-throughput sequencing to identify inherited alterations in those genes associated with higher risk of certain cancers. Results of the test are intended to be interpreted by professionals for discussion with patients, bringing in factors such as family and personal history. It is not designed for direct-to-consumer reporting, nor does the test detect predisposition to all known cancer risks.
Among the variants detected by the Common Hereditary Cancers Panel are BRCA1 and BRCA2, associated with breast and ovarian cancer. Also reported are several genes associated with risk of Lynch syndrome, an inherited form of colorectal cancer and other solid tumor cancers. Invitae says, in addition, the genetic alterations revealed by the test indicate risk of developing certain forms of uterine, prostate, stomach, and pancreatic cancer.
99 percent or higher accuracy
The company says physicians can also use the Common Hereditary Cancers Panel determine if patients already diagnosed with cancer harbor any of the 47 inherited gene variants. FDA notes, however, that this and other tests of its kind run risks of false positive or negative results, leading to inappropriate medical decision-making or a false sense of assurance. Nonetheless, says FDA, Invitae tested the panel with more than 9,000 clinical samples, achieving 99 percent or higher accuracy on all of the variant types assessed.
“Today’s action can provide an important public health tool,” says Jeffrey Shuren, director of FDA’s Center for Devices and Radiological Health in an agency statement, “that offers individuals more information about their health, including possible predisposition for certain cancers, which can help guide physicians to provide appropriate monitoring and potential therapy, based on discovered variants.” Shuren adds, “This test can assess multiple genes in a single test by using next-generation sequencing, which has proven helpful in providing insight into genetic variants with sensitivity and speed.”
FDA says the Common Hereditary Cancers Panel is the first test of its kind to receive marketing clearance. FDA also issued special controls for labeling and performance testing of the test. The agency says it authorized the test under the agency’s De Novo marketing pathway, a mechanism for completely new types of medical devices rated low or moderate risk. As a new form of medical device, the Common Hereditary Cancers Panel can serve as a so-called predicate device that future genetic tests of its type can compare for pre-market authorization from FDA.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|










 RSS - Posts
RSS - Posts
You must be logged in to post a comment.