Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on December 28th, 2019 – Contributed content –
 (Riccardo Bresciani, Pexels) 28 Dec. 2019. Logistics companies have a massive potential for profit. They are a vital service in the business world. Many companies rely on the services of logistics companies and are able to do cut out big costs from their own business model. It’s important that you are providing a modern solution with your logistics company and it should come as no surprise that tech is providing the answer.
Going green
You should definitely consider the tech that is going to make your business more green this year. First, do think about the warehouse. You’re going to be using a lot of vehicles and equipment to move stock around. It is possible to explore green-friendly options that are going to save you a fortune in energy and help keep your business on a modern trajectory.
You also need to consider the vehicles that you are going to use to deliver your products to the buyers. There are many options on the market here but you definitely need to focus on hybrid and electric. Hybrid provides the best of both worlds, allowing you to save on energy with the power and range of a typical engine.
In contrast, electric is perhaps the greenest option available but won’t be suitable for long haul projects. If you need to deliver packages within the city, it could be a great choice. However, for packages across state lines, this is going to be both expensive and time-consuming.
When exploring the full business model, it might also be worth looking into options like renewable energy. If you own your warehouse, you could consider investing in renewable sources of energy for these buildings. This would provide a massive benefit and ensure that you could see the fantastic level of savings you want. Through the summer months, you might see little to no electricity usage.
This tech is also advancing quickly. You can now store energy collected from these to use it when you have the greatest possible need. This could be incredibly beneficial if you have noticed the costs of running your business are too high.
Automation
Automation is best described as any process in a business that will not require human interaction. As you might have guessed, limiting the level of human interaction will also limit the potential for human error. We think that you’ll agree this is certainly good news.
It puts your business in a stronger position on the market because you will be delivering greater service to consumers or clients. You won’t, for instance, need to worry about issues with the product not being delivered to the right client.
Automation can be implemented into various levels of the logistics company. Right now, the main point of focus should be the warehouse. With automation, you will require a lower number of workers to complete the same processes which are great news. As well as this, you will be able to make sure that you are able to keep processes in the warehouse moving forward more efficiently without issues with delays or downtime.
Something to keep an eye on is the automated technology that is entering the automobile industry. It won’t be too long before we see the first autonomous trucks on the market which will be fantastic for logistics companies.
These trucks are going to ensure that the roads are safer while keeping deliveries efficient. Again, it’s going to tackle that issue of human error. You will still need to employ drivers because this tech will require a backup. However, for the most part, a computer will handle the driving. Trucking accident injury law problems can cost companies hundreds of thousands and this can save you from that problem.
With automation, you won’t just be making your business more valuable to your customers. You will be saving money in your business model as well. It could change the game completely and help ensure that you stay ahead of the competition on the market.
Tracking technology
You should make sure that you are using tracking tech in your logistics business. Unlike automated technology, you won’t have to wait for this tech to emerge. It’s available on the market right now and we guarantee your competitors are already accessing it.
What does tracking allow? With tracking, you will be able to make sure that you can keep a check on what is happening with individual deliveries. You will know where they are on the route and when they will be delivered to the client. You will also be able to use the data to make clear predictions about future routes. This can help you give accurate estimated delivery times. That will be particularly important if a large portion of your client base is B2B companies. They will need to be able to ensure that deliveries are not just rapid but reliable. Particularly, when the supplies they have ordered could be a crucial part of their business model.
Of course, there’s another benefit to tracking tech. You will be able to share with clients exactly when a package is going to arrive. They will certainly thank you for this because it means that they will be able to plan around the delivery. Polls have revealed that a customer is more likely to order if they know that tracking information is available.
You might think that this type of technology is out of your price range. However, you would certainly be surprised by the affordability of options like this. It’s definitely not the massive cost that it used to be for companies. Instead, you could find it will be one of the cheapest investments that you will make in your business. Particularly if you buy early.
We hope this helps you understand the biggest benefits tech can bring to a logistics company. Tech is pushing the business world to new heights. It’s crucial that if you are operating in the logistics industry, you are not left behind. There is massive potential here and it’s all but a guarantee that your competitors are using some of these great options.
Editor’s note: The opinions in this post are the contributor’s and not those of Science & Enterprise.
* * *
By Alan, on December 27th, 2019  Natural killer cell (NIAID, Flickr) 27 Dec. 2019. A biotechnology company creating synthetic proteins that invoke the immune system to fight cancer is being acquired by drug maker Astellas Pharma. The deal with Astellas Pharma Inc., in Tokyo, is expected to bring shareholders of Xyphos Biosciences, in South San Francisco, California as much as $665 million if all aspects of the agreement are fulfilled.
Xyphos Biosciences, a three year-old company, develops synthetic proteins based on natural human receptor proteins known as NKG2D. These proteins appear on the surface of natural killer and T-cells in the immune system, and are considered key receptors for detecting and fighting damaged or pathogen-infected cells, including tumor cells. The Xyphos technology, called Advanced Cellular Control through Engineered Ligands or Accel, takes little-used ligands, or connector molecules, in NKG2D proteins and modifies their chemistry to provide highly specific targets, while deactivating other ligands.
The Xyphos technology then adds immunotherapy proteins that invoke the immune system to find and kill cancer cells. Among these immunotherapies are T-cells in the immune system with chimeric antigen receptors added, called CAR T-cells, to target tumor tissue. Xyphos says that unlike earlier CAR T-cells, its engineered CAR T-cells are more precisely targeted and controlled, with longer life cycles in the body and fewer adverse effects. All of the company’s therapy candidates are either in preclinical or discovery stages.
Astellas has a number of cancer drug candidates, including immunotherapies, in clinical trials, but plans to use the Xyphos/Accel technology to develop a new type of cancer treatments. “The innovative technology in development at Xyphos,” says Kenji Yasukawa, president and CEO of Astellas in a company statement, “fits perfectly in advancing our immuno-oncology strategy to create and deliver value for patients. Combining this technology with our capabilities in cell therapy that we have been working on so far, we can create next-generation high-function cells and maximize the value of our technology.”
Under the agreement, Xyphos becomes a wholly-owned subsidiary of Astellas. Xyphos is receiving an initial payment of $120 million from Astellas, with additional milestone payments of $545 million expected.
More from Science & Enterprise:
* * *
By Alan, on December 26th, 2019  Scanning electron micrograph of methicillin-resistant Staphylococcus aureus, or MRSA, in brown spheres, surrounded by cellular debris. (NIAID, NIH) 26 Dec. 2019. Readers of yesterday’s New York Times woke up to disturbing news of difficult science and failing business models for stemming the scourge of infections resistant to antibiotics. Solving this vexing problem may take a different way of treating infections, with a new type of business to develop and produce the treatments.
Yesterday’s story reveals more of a business than scientific failure. Times health and science reporter Andrew Jacobs tells about antibiotic drug makers going bankrupt even as some products show promise against drug-resistant bacteria. And even with government contracting for these new antibiotics, venture investors are reluctant to bankroll developers of these new drugs, preferring bigger payoffs from treatments for chronic disorders like cancer and heart disease.
Yet as the story also notes, the science of finding new antibiotics isn’t getting any easier. Jacobs reports that in the last 20 years, only two new classes of antibiotics have reached the market, with most new drugs designed as variations of current drugs. And the difficult science contributes to a high price tag for developing new antibiotics, as much as $2.6 billion, that’s driving away many pharma companies, leaving only three drug makers today in the antibiotics market.
The story notes that the U.S. government is using its available tools and resources to find a solution. An agency leading this effort is the Biomedical Advanced Research and Development Authority, or BARDA, part of the Health and Human Services department. BARDA supports research and contracts for stockpiles of drugs to prevent or treat public health and bio-terrorism threats, including drug-resistant microbes. The agency also supports an international consortium called the Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator, or CARB-X to develop new antibiotics. But even with BARDA buying $124 million of its lead drug, antibiotics developer Achaogen, a company with a market capitalization at one time of $1 billion, still went bankrupt.
Precision medicine for antibiotics
One of the reasons for the difficult science and high cost of antibiotics may be the way the drugs designed and made. The goal of many new antibiotics is to find a mechanism that kills the microbe or disables its infecting process. And the more bacteria or fungi the antibiotic can neutralize, the more desirable a product it becomes, both medically and financially. But microbes often evolve quickly to evade these mechanisms, making antibiotic development a constant race against nature to find these silver bullets, requiring continuous R&D, a reason why many investors are reluctant to back antibiotics developers.
This suggests a need for a different approach to fight drug-resistant bacteria and fungi. Many parts of a new model for fighting antibiotic resistance are in development, and the pieces still need to be assembled, but the outline of a precision-medicine rather than mass-produced drug model is beginning to emerge.
In January, Science & Enterprise reported on a lab at Johns Hopkins University that uses genomic sequencing to identify vulnerabilities in a bacterium’s DNA. A team led by pathologist and microbiologist Patricia Simner uses a hand-held genomic sequencing device called called the Minion made by Oxford Nanopore Technologies in the U.K. The Minion is a portable disease surveillance system that analyzes DNA from blood samples, returning results in as little as an hour, and operates as a plug-in peripheral on a laptop computer.
The Minion technology forces single strands of DNA through nanoscale pores, which makes it possible to analyze samples in real time. Simner and colleagues found they can analyze the whole genomes of Klebsiella pneumoniae, a gram-negative bacteria associated with pneumonia, blood stream infections, wound or surgical site infections, and meningitis, sampled from 40 patients. And, the team reassembled the bacterial genomes, identifying target genes and mutations for antibiotics, all in about one day, with 92 percent accuracy.
This is a different take on precision medicine, where in this case genomic sequencing identifies vulnerabilities of microbes rather than disease-causing mutations in patients. Nonetheless, it offers precise targets in real patients’ infections for drugs, rather than the trial-and-error approach to treating infections often used today.
Diagnostics, of course, is only a first step in the process. Precise formulations are needed to hit the precise targets identified by the genomic sequencing. In the past two years, we’ve seen rapid advances in computational biology and artificial intelligence applied to drug discovery, many of which we’ve documented on this site.
For antibiotics in particular, we reported in October 2018 on biochemistry professors Christophe Corre and Manuela Tosin at University of Warwick in the U.K. that use bioinformatics and the genome-editing technique Crispr to develop a new antibiotic for tuberculosis bacteria. The Warwick team started with naturally-occurring microbes in soil, then altered the genome with Crispr to remove a genetic barrier to produce therapy candidates. The researchers needed several rounds of Crispr editing to find the eventual solution, but the authors say the process can be automated.
One of those rapidly advancing automated tools applied to drug discovery is artificial intelligence, particularly deep learning techniques. In September, we reported on a paper in the journal Nature Biotechnology where researchers from Insilico Medicine in Hong Kong and WuXi AppTec in Shanghai devised deep learning techniques to quickly discover small-molecule drugs. In this case, the researchers discovered potential treatments for blocking an enzyme associated with cirrhosis, or scar tissue in the liver, and other diseases. With this technology, the team discovered drug candidates, ready for validating and preclinical animal testing in 21 days. Later tests with lab animals verified the treatment candidates’ chemical activity.
Small-batch production and distribution
With precision medicine comes a need for smaller quantities of drugs produced on demand, which may not be possible with large-scale bio-reactors that need long lead times. In June 2017, we reported on an initiative by drug maker Eli Lilly and Company, published in the journal Science, about a process for making small quantities of drugs on demand using continuous flow manufacturing processes. The researchers succeeded in producing quantities of a cancer drug as small as three kilograms a day for eight days with techniques that meet current good manufacturing practices. The technology, say the authors, uses advances in chemistry, engineering, analytical science, process modeling, and equipment design.
About a year earlier, we reported on a paper in the journal Nature Communications about a portable, small-batch production unit for biologic drugs. Like chemical compounds, biologic drugs normally need long lead times and are produced in large batches, but Defense Advanced Research Projects Agency, or Darpa, asked a group led by MIT bio-engineering professor Timothy Lu to develop a small-scale bio-reactor. The reactor, measuring 31 x 34 x 36 centimeters, uses microfluidics and a strain of genetically engineered yeast to produce single-dose quantities of two biologic drugs in less than 24 hours.
Fast delivery of small packages in the U.S. is amply demonstrated almost every day, but getting drugs to remote locations is still a challenge. In July, we reported on a project by the humanitarian organization Direct Relief and drone maker and delivery company Volans-i Inc., to deliver drugs needing refrigeration to remote locations. The project team, including drug maker Merck, successfully delivered a drug package in refrigerated boxes between islands in the Bahamas, crossing open water, and well beyond the line of sight of the dispatch team.
A new type of business
Bringing together all of these pieces will likely need a new kind of business, since most of today’s pharmaceutical companies and investors show little interest in antibiotics. We reported in November on a consortium taking shape in Massachusetts that may provide a model for this business. The not-for-profit consortium is made up of companies, universities, and hospitals to create an innovation and manufacturing center in the Boston area for cell and gene therapies. The center is expected to provide lab and production facilities for genome editing, stem cells, and immunotherapies. The goal is to break bottlenecks that impose delays as long as 18 months in ramping up production for preclinical studies and clinical trials.
In May, we wrote about ElevateBio, a company in Cambridge, Massachusetts offering full-scale facilities to develop cell and gene therapy products spun-off from academic research labs. ElevateBio is expected to have a central product development lab and manufacturing facility for gene and cell therapies to be shared among ElevateBio’s portfolio companies. The facility aims to provide automated protein engineering, virology, and immunology labs, as well as analytics and quality-control resources.
Both of these new enterprises are located in the biotechnology hotbed of Boston, but similar lab and manufacturing facilities will be needed throughout the country. A nationwide effort will likely require a combination of private and public investments, with federal money making up most of the public funds. One model to consider for these facilities are the national laboratories, part of the U.S. Department of Energy, but operated by private contractors.
The need for new drugs to treat infections is increasing, while current methods for finding and producing these drugs are disappearing. We need to start now, beginning with new precision-medicine processes to find those drugs, and new business models to get them in the hands of clinics.
* * *
By Alan, on December 25th, 2019  (A. Kotok) 25 Dec. 2019. It’s Christmas day and a holiday in many countries around the world, including the U.S. So we will not publish today. If you celebrate Christmas, or other holidays this time of year, enjoy the celebration. Science & Enterprise will return tomorrow, 26 December.
* * *
By Alan, on December 24th, 2019  Tomatoes genetically modified to grow faster and in bunches (Lippman Lab, Cold Spring Harbor Laboratory) 24 Dec. 2019. Plant scientists used a genome-editing technique to produce varieties of tomatoes better suited for growing on urban farms and other small plots. Researchers from Cold Spring Harbor Laboratory in Long Island, New York and other institutions describe their plants in yesterday’s issue of the journal Nature Biotechnology (paid subscription required).
A team led by CSHL plant geneticist Zachary Lippman is seeking food crops that can be grown quickly in limited spaces, such as urban community farms and green roofs, while still retaining their desirable taste and color qualities. The authors note that growing crops in these smaller plots and closer to customers, can have beneficial environmental impacts, particularly with many traditional rural farms threatened by climate change. Up to now, however, only green leafy vegetables have been shown to grow readily on urban farms.
Lippman and colleagues in the U.S., Germany, Korea, and Israel took a different approach, by designing crops with flowers and fruits with properties needed to thrive in urban environments. These properties include smaller spaces and less time for growing, which allows for more harvests per year. These qualities, however, are basic features of these crop plants. “When you’re playing with plant maturation,” says Lippman in a CSHL statement, “you’re playing with the whole system, and that system includes the sugars, where they’re made, which is the leaves, and how they’re distributed, which is to the fruits.”
The researchers identified a series of traits for modification in Solanaceae plants, which cover a range of common flowering and crop plants, including peppers and tomatoes. The team used the genome-editing technique Crispr to alter specific genes in vine tomatoes. Crispr — short for clustered, regularly interspaced short palindromic repeats — makes it possible to edit genomes of organisms by harnessing bacterial defense mechanisms that use RNA to identify and monitor precise locations in DNA.
Among the tomato genes edited by the lab are SlER that regulates the length of stems and vines, SP5G for reducing the time needed for flowering, and SP to terminate growth earlier. The researchers discovered they could alter these genes in one step with Crispr, resulting in compact, quick-growing plants more suitable for urban farms, with fruit that grow in bunches more like grapes. The team confirmed the viability of these varieties in greenhouse tests, including maintaining crop yields with the smaller plants.
“This demonstrates how we can produce crops in new ways,” says Lippman, “without having to tear up the land as much or add excessive fertilizer that runs off into rivers and streams.” He adds, “They have a great small shape and size, they taste good, but of course that all depends on personal preference.”
The Lippman lab’s work is covered by current and applied patents, and exclusively licensed to Inari Agriculture in Cambridge, Massachusetts. Inari Agriculture is a four year-old company using genetics and and bioinformatics to create more sustainable and environmentally-friendly crops. Lippman is a scientific adviser, and co-author Zachary Lemmon, a former postdoctoral researcher at CSHL, a bioinformatics scientist at Inari Agriculture.
More from Science & Enterprise:
* * *
By Alan, on December 23rd, 2019  (Gerd Altmann, Pixabay) 23 Dec. 2019. A new paper proposes an accounting standard that would require private companies to spell out their costs of meeting net-zero greenhouse gas emissions. Economists Richard Murphy at City University of London and Leonard Seabrooke at Copenhagen Business School in Denmark describe their proposal in today’s issue of the Danish journal Samfundsøkonomen (Society’s Economist).
Murphy, also a chartered accountant — or certified public accountant in the U.S. — and Seabrooke are seeking a feasible and standard method for companies to report the costs they expect to face in meeting the impending climate crisis. The authors note that the debate over mitigating the effects of climate change often revolves around actions by government, such as the so-called Green New Deal, with little discussion given to the impact on businesses of transitioning to a net-zero emissions economy.
To make companies better aware of the costs they face from the climate crisis, as well as financial institutions supporting these enterprises, Murphy and Seabrooke propose a sustainable cost accounting standard. This standard would report to financiers and other corporate stakeholders — employees, trading partners, regulators, tax authorities, and civil society as a whole — the issues imposed by climate change on the company and geographic locations where those issues affect the company. In addition, the standard calls for detailing the impacts of climate change at those company locations, response in each community to those challenges, and time schedule for addressing those issues.
Up to now, costs of climate change on corporations are largely proposed in terms of government-imposed taxes or fees. The sustainable cost accounting standard, or SCA, however would be built into a company’s accounting practice. “As a standard,” say the authors, “SCA would build on existing accounting principles to require commercial organizations to report on how they will manage the costs of becoming net carbon-zero compliant. SCA does not include carbon pricing or the cost of offsets. It would require the commercial organization to establish the costs of the transition to carbon neutrality.”
The authors note that other international groups, including the Task Force on Climate-Related Financial Disclosures, issued financial reporting guidelines for costs of climate change, beginning in 2017. But while 80 percent of the world’s largest corporations endorse the guidelines, a review of their implementation shows fewer than a quarter of the enterprises do any meaningful reporting on costs imposed by climate change. As a result, say the authors, sustainable cost accounting standards need to be fully integrated into routine audited financial reporting requirements, and not be an add-on feature.
Murphy and Seabrooke say that the implementation of a sustainable cost accounting standard would for many large companies be a straightforward exercise, adapting current accounting measures and techniques to a different target: costs of reaching zero-net emissions of greenhouse gases. The sustainable cost accounting standard would also apply to suppliers to large companies, which would encourage implementation by smaller businesses. And calculating costs of meeting net-zero emissions would assume use of existing technologies, say the authors, which would also encourage development of new more productive technologies.
If by applying a sustainable cost accounting standard, a company finds it cannot meet the goal of net-zero emissions, it would declare itself in a state of carbon insolvency, requiring a plan to structure its finances accordingly. This restructuring, say Murphy and Seabrooke, may require reducing dividend payments or raising additional capital. Continued carbon insolvency would call into question whether the company meets the definition of a going concern, raising alarms about its future economic viability.
More from Science & Enterprise:
* * *
By Alan, on December 23rd, 2019  T-cell infected by HIV (NIAID, Flickr) 23 Dec. 2019. The U.S. Food and Drug Administration rejected a combination of experimental and existing drugs given once a month to treat HIV. The application to FDA was made to approve a single monthly injection of the experimental drug cabotegravir by ViiV Healthcare in London, and the current HIV drug rilpivirine, marketed as Edurant by Janssen Pharmaceuticals, a division of Johnson & Johnson, in Titusville, New Jersey.
ViiV Healthcare and Janssen say FDA expressed its concerns in a complete response letter, correspondence for rejecting a new drug application to the agency. ViiV — a joint venture of drug makers GlaxoSmithKline, Pfizer, and Shionogi to develop HIV drugs — and Janssen say FDA raised objections to proposed chemistry, manufacturing, and controls processes for the combination drug. These processes often involve a product’s identity, strength or potency, quality, and purity. The companies point out that FDA did not raise any safety issues with the proposed drug combination.
Cabotegravir is under development by ViiV as both an oral drug and long-acting injectable treatment for HIV. The drug blocks an enzyme called integrase that inserts DNA from HIV viruses into white blood cells in the immune system. By blocking this integration of viral DNA into immune system cells, HIV cells cannot multiply and the amount of HIV is reduced in the body.
Rilpivirine, or Edurant, is already approved by FDA as a daily therapy for HIV, but is prescribed for certain teens and adults with HIV only when taken with other drugs. Rilpivirine blocks an enzyme called reverse transcriptase that prevents HIV from multiplying, and thus reduces the amount of HIV in the body.
ViiV and Janssen tested the two drugs, formulated into an injectable treatment given once a month, in a series of clinical trials. In a late-stage trial reported in October 2018, the injected cabotegravir and rilpivirine combination given once a month shows the same efficacy as a daily three-drug combination among adults who already succeeded in reducing their HIV levels. Results from another late-stage trial reported in August of this year shows the cabotegravir-rilpivirine combination injected every eight weeks works as well as the drugs given every four weeks.
ViiV Healthcare and Janssen say they will work with FDA to determine the appropriate next steps for advancing this HIV treatment. As reported by Science & Enterprise in April 2019, FDA approved a two-drug combination brand-named Dovato by ViiV Healthcare, as a daily oral drug to lower HIV viral loads.
More from Science & Enterprise:
Disclosure: The author owns shares in Johnson & Johnson and Pfizer.
* * *
By Alan, on December 21st, 2019 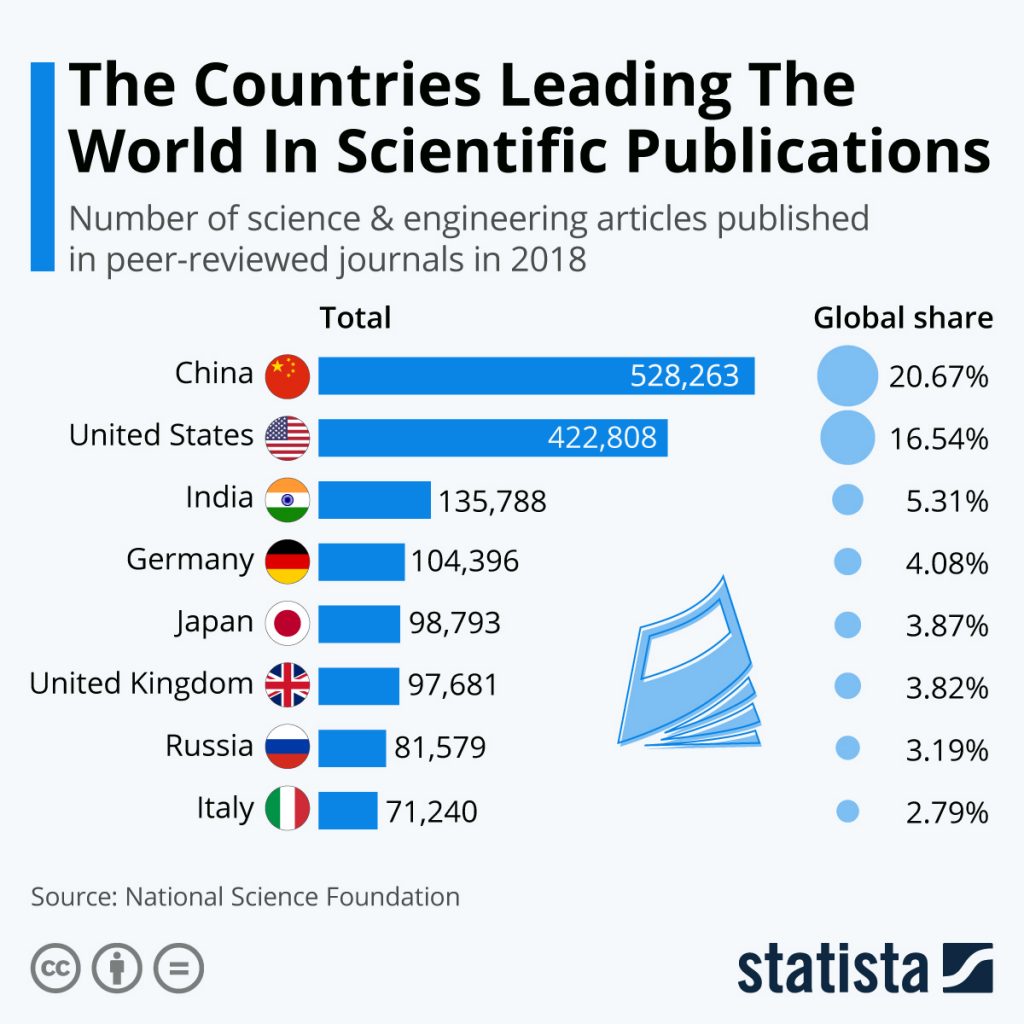 Click on image for full-size view (Statista) 21 Dec. 2019. China now leads all other countries in the sheer output of papers in peer-reviewed scientific journals, according to data from National Science Foundation. The findings, displayed yesterday by our friends from Statista, is the Science & Enterprise infographic for this weekend.
The NSF report, part of the agency’s Science and Engineering Indicators series, shows more than 528,000 scientific papers from China were published in 2018, accounting for 21 percent of the total, eclipsing the nearly 423,000 papers from the U.S. that account for 16.5 percent. India follows with 136,000 and Germany with 104,000 publications. Taken together, however, European Union countries published some 622,000 scientific papers, about a quarter of all papers in 2018, and exceeding the number from China.
NSF says research output worldwide over the past 10 years, measured in journal articles and conference papers, expanded at a rate of 4 percent per year. China turns out scientific papers at twice the rate of the rest of the world, while the U.S. and European Union countries publish papers at about half of the worldwide rate. And while the U.S., Japan, and EU countries publish more research from the life sciences and health, China and India tend to publish more studies on engineering.
More from Science & Enterprise:
* * *
By Alan, on December 21st, 2019 – Contributed content –
 (Pexels.com) 21 Dec. 2019. If we’re completely honest, the vast majority of people are kind of selfish. Now that’s not the same thing as saying that someone is a bad person or that someone doesn’t care about anyone else. But it does mean that most of us spend our time in our own little bubble, mostly only concerned with the things that affect us directly. This is important, being able to take care of yourself is one of the most important things there is.
But for many of us, that leaves us feeling like there is more that we could be doing. A lot of people would love to be able to find a way to give back, to improve the world that they live in, even if it’s just little bit. Well, fortunately, you can. It doesn’t need to be in huge ways, but there are some simple things that you can do to actually help other people around you.
Volunteer
Charities and nonprofit organizations are always looking for enthusiastic volunteers to help them out. If there’s a charity that you support and you want to go that extra step further, contact them and ask what you can do. This could mean getting involved in activities that they are doing, fundraising or getting out there on the front lines with the people that the charity is trying to help. It might not feel that one extra person can do much good but even the slightest extra support that these kinds of organizations can get can make an enormous difference.
Learn first aid
No situation can make you feel more powerless than when someone collapses, and you have no idea what to do. You just stand there, frozen in panic while other people rush in to help. The only way to get past that panic and to become one of those people trying to help is by knowing what to do. There are plenty first aid courses available as well as tutorials online. They’ll teach you the correct way to perform CPR, what to do if someone’s heart stops and things like how to use a defibrillator. That way, if you’re ever in a position where someone has an accident, instead of being frozen and feeling totally useless, you can actually do something to help.
Change your career
Let’s say you want to make a larger, more permanent difference. Then why not change your career completely to pursue something that allows you to help people? A career in medicine is a fantastic opportunity to do that. Don’t assume that you need to study for years and become a doctor either. There are plenty of medical professions that you can come into with far less training. You also have the option of actually going to work for a charity. People like https://www.wopg.org/prem-rawat/ have built their entire lives and careers out of helping others. Working as part of those kinds of organizations can be an incredible experience. That way, instead of being an occasional help, you become an integral part of the organization.
Remember, even when it feels as though there isn’t anything you can do on your own, you’re one of millions of people who are trying to do their best and help others. And that can make a world of difference.
* * *
By Alan, on December 20th, 2019  HaptX glove (HaptX Inc.) 20 Dec. 2019. A company making gloves that transmit a sense of touch in games and commercial robotics is raising $12 million in its first venture funding round. HaptX Inc. in Seattle is also partnering with Advanced Input Systems, a developer of human-machine interface technologies,in Coeur d’ Alene, Idaho.
HaptX develops systems that provide realistic touch feedback, built in a glove with sensors and skins to capture, transmit, and receive haptic signals. The glove contains a thin (1.5 millimeter) smart textile skin with panels of air channels and 130 pneumatic actuators that convey haptic feedback to the wearer. A lightweight exoskeleton is built on the glove to provide up to four pounds of force on each finger that the company says complements the glove’s smart textile skin.
In addition, the glove system uses software that tracks precise motions and position of the hand. HaptX says the hand tracking software offers sub-millimeter accuracy, with an automatically generated and individualized hand model. A silent mini-compressor provides air to the pneumatic skin and actuators, regulated with a compact control panel, which the company says can operate in an office setting.
HaptX makes its haptic glove for virtual-reality games, as well as training systems using virtual reality. The company is collaborating with car maker Nissan to develop virtual-reality systems with haptic feedback to help automotive design engineers interact with 3-D car models. HaptX says the technology can reduce the need for multiple full-scale prototypes.
The company’s first venture funding round is raising $12 million from existing investors NetEase and Amit Kapur of Dawn Patrol Ventures, with proceeds aimed at creating the next generation of haptic gloves. Joining the round are new investors Mason Avenue Investments, Taylor Frigon Capital Partners, Upheaval Investments, Votiv Capital, Keiretsu Forum, and Keiretsu Capital. HaptX, founded in 2012, previously raised $7 million in seed funds.
HaptX also announced a collaboration with Advanced Input systems, a maker of human-machine interface technologies that it says are used in medical, industrial, defense, and gaming industries. Eric Ballew, president of Advanced Input Systems, says in a HaptX statement, “HaptX and Advanced Input Systems are overcoming a long-standing technology gap of seamlessly connecting the physical world to the virtual world.”
The collaboration is expected to span product development, manufacturing, and marketing, but financial and intellectual property details were not disclosed.
More from Science & Enterprise:
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|











 RSS - Posts
RSS - Posts
You must be logged in to post a comment.