Janssen Pharmaceutical Companies, a division of Johnson & Johnson in Horsham, Pennsylvania, agreed to license a cancer drug targeting multiple myeloma made by biotechnology company Genmab A/S in Copenhagen, Denmark. The agreement has a total potential value of $1.1 billion from upfront, milestone, and royalty payments, as well as an equity investment in Genmab.
The agreement gives Janssen a worldwide license to develop and commercialize daratumumab, a monoclonal antibody that targets the CD38 molecule which is highly expressed on the surface of multiple myeloma cells. In pre-clinical studies, says Genmab, daratumumab killed multiple myeloma cells and enhanced the potency of other multiple myeloma treatments. Daratumumab is now in phase 1 and 2 clinical trials, testing the drug’s safety for treating multiple myeloma, both on its own and when given with other cancer drugs, lenalidomide and dexamethasone.
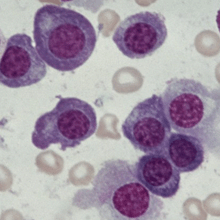
Multiple myeloma is a cancer that causes the plasma cells in bone marrow to grow out of control and form tumors in the areas of solid bone. The growth of these bone tumors then makes it harder for the bone marrow to make healthy blood cells and platelets.
Genmab says daratumumab has potential as a treatment for other types of cancers. The company says daratumumab could be applied to other tumors where the CD38 molecule is expressed, including diffuse large B-cell lymphoma, chronic lymphocytic leukemia, acute lymphoblastic leukemia, acute myeloid leukemia, follicular lymphoma, and mantle cell lymphoma.
Under the agreement, Janssen made an upfront payment of $55 million and will make additional payments based upon the achievement of development, regulatory, and sales milestones. Genmab values those milestones and subsequent royalties at about $1 billion. Janssen will be responsible for further development and commercialization costs for daratumumab, including the two current clinical studies.
Also part of the deal is an equity investment by Johnson & Johnson Development Corporation of some 5.4 million new shares of Genmab stock worth about $80 million. After the issue of these shares, Johnson & Johnson will own 10.7 percent of Genmab’s share capital.
Read more:
- Biotech Developing Anti-Cancer Drugs Gets $15M Investment
- Early Trial Evaluates Drug Combo for Blood Cancers
- Myeloma Foundation Awards Biotech Research Grants
- Trial Shows Results for Multiple Myeloma Therapy
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.