Emergent BioSolutions Inc. in Rockville, Maryland says that its program for developing AVP-21D9, an antibody for the treatment of inhalational anthrax, has been granted Fast Track designation by the U.S. Food and Drug Administration (FDA).
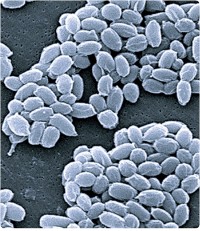
Anthrax commonly affects hoofed animals such as sheep and goats, but also humans who come into contact with the infected animals. In the past, the people who were most at risk for anthrax included farm workers, veterinarians, and tannery and wool workers. Inhalation anthrax develops when anthrax spores enter the lungs through the respiratory tract. Anthrax can be used as a biological weapon or for bioterrorism.
Emergent began last month a Phase 1 clinical trial for AVP-21D9, which is a fully human monoclonal antibody candidate being developed as a parenteral (intravenous or injected) post-exposure therapeutic to treat symptoms of inhalational anthrax disease. The trial involves 50 healthy volunteers in a randomized, double-blind, placebo-controlled, dose escalation study designed to evaluate the safety and pharmacokinetics of the candidate.
FDA’s Fast Track process provides for expedited regulatory review of drugs and biologics that treat serious or life threatening diseases and demonstrate the potential to address unmet medical needs.

 RSS - Posts
RSS - Posts
[…] FDA Fast-Tracks Antibody for Inhalational Anthrax Treatment […]
[…] Related: FDA Fast-Tracks Antibody for Inhalational Anthrax Treatment […]