Talecris Biotherapeutics, a biotechnology company in Research Triangle Park, North Carolina said today that the U.S. Food and Drug Administration (FDA) approved its Gamunex-C drug for subcutaneous administration in the treatment of primary immunodeficiency (PI).
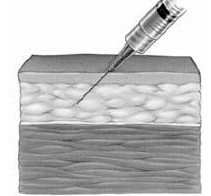
Subcutaneous administration — illustrated right — delivers the product under the skin into the subcutaneous tissue and intravenous administration is delivered through a vein. Gamunex-C provides for both the intravenous and subcutaneous administration. The subcutaneous mode is approved to treat only PI. The intravenous delivery mode is approved to treat PI, chronic inflammatory demyelinating polyneuropathy — damage to the myelin sheath of the peripheral nerves — and idiopathic thrombocytopenic purpura, a lack of blood clotting due to a low number of blood platelets.
Gamunex-C is an extension of Talecris’s Gamunex product, which is made only for intravenous administration, while Gamunex-C is available for both subcutaneous and intravenous delivery.
Primary immunodeficiency diseases occur in persons born with an immune system that is either absent or hampered in its ability to function. While not contagious, these diseases are caused by hereditary or genetic defects and can affect anyone, regardless of age or sex. The World Health Organization recognizes more than 150 PI diseases.

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.