29 May 2014. The U.S. Food and Drug Administration yesterday approved an implanted device that measures pulmonary artery pressure and heart rate of patients with moderate heart failure who were hospitalized in the previous year. The device, the CardioMEMS HF System, is made by CardioMEMS Inc. in Atlanta.
St. Jude Medical, a medical device manufacturer in St. Paul, Minnesota, owns 19 percent of CardioMEMS, with an option to acquire the rest of the company. St. Jude Medical says it intends to exercise that option, and purchase the remaining 81 percent of CardioMEMS for $375 million during the second quarter of this year.
Heart failure is a condition where the heart cannot pump enough blood to meet the body’s needs, a condition affecting some 5.8 million people in the U.S. In cases of worsening heart failure, physicians measure pressure in the pulmonary artery that pumps blood from the heart to the lungs, where the blood is enriched with oxygen. Increased pressure in the pulmonary artery is an early sign of worsening heart failure, often requiring intervention.
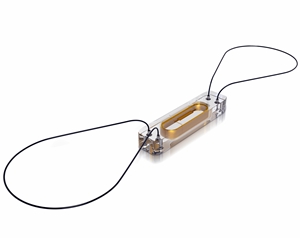
The CardioMEMS device is a combination miniaturized sensor and monitor implanted in the pulmonary artery with a catheter inserted through a vein. The sensor/monitor emits a signal picked up by an antenna held outside the body over the implant site, and transferred to a collection station. Readings taken daily by the patients are then sent via secure communications to the physician for review and analysis.
FDA based its approval in part on a clinical trial with 550 patients having moderate heart failure, defined as causing marked limitations on physical movement, where ordinary activities result in fatigue or shortness of breath. The patients, all of whom were previously admitted for heart failure, were either implanted with a CardioMEMS device or received the standard care. The researchers used readmission to the hospital for heart failure as the main measure of the device’s effectiveness.
The results, published in the journal The Lancet, show in the first 6 months after the implants, the CardioMEMS patients reported a statistically reliable lower rate of readmission for heart failure (31%) than those receiving the standard care (43%). During the follow-up period of 15 months, patients with the CardioMEMS system also had a 37 percent lower hospital readmission rate. The researchers reported 8 cases of device or system complications in the 270 implants, and no incidents of failure of the implanted sensor.
FDA approved the device for patients similar to those in the clinical trial: those with moderate heart failure and were hospitalized for the condition in the previous year. The agency is also requesting a thorough post-approval study of the system’s performance outside the controlled environment of a clinical trial.
Hospitals now have more incentives to reduce readmissions for heart failure. A provision of the Affordable Care Act penalizes hospitals if their readmission rates for heart attack, heart failure, or pneumonia exceed national averages.
Read more:
- Wireless Power System Invented for Miniaturized Implants
- Remote Device Monitoring Linked to Higher Survival Rates
- Injectable Hydrogel Developed to Prevent Heart Attack Damage
- Early Trial Shows Wireless Pacemaker Safe, Effective
- Implanted Heart Membrane Device Created by 3-D Printer
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.