13 Feb. 2019. An organization advocating for medicines addressing a person’s unique chemical composition rather than disease symptoms says U.S. regulators approved 25 of those drugs in 2018. The Personalized Medicine Coalition in Washington, D.C. says in a report released today these therapies represent more than 4 in 10 of the new drugs approved by the Food and Drug Administration last year.
Personalized or precision medicines are those targeting the underlying chemistry of proteins expressed by an individual rather than the state of a disease. In October 2018, FDA issued regulatory guidance on therapies addressing proteins acting as indicators, known as biomarkers, often a result genetic changes such as mutations, which may be associated with more than one disorder. Also in some cases, conventional one-size-fits-all drugs may address these disease-causing proteins, but affect other functions in the body as well, resulting in undesired side effects.
FDA lists 59 new molecular entities — a term covering both chemical drugs, agents, and biologic therapies — approved in 2018. Personalized Medicine Coalition rates 25 of those new treatments, or 42 percent, as personalized medicines. Of the 25 approvals, 10 targeted biomarkers associated with cancer. Included in these 10 new therapies is Vitrakvi made by Loxo Oncology Inc. in Stamford, Connecticut addressing a molecular condition resulting in cancer-causing proteins that encourage solid tumor onset, growth, and development in various parts of the body. In January of this year, as reported by Science & Enterprise, Eli Lilly and Co. bought Loxo Oncology for some $8 billion.
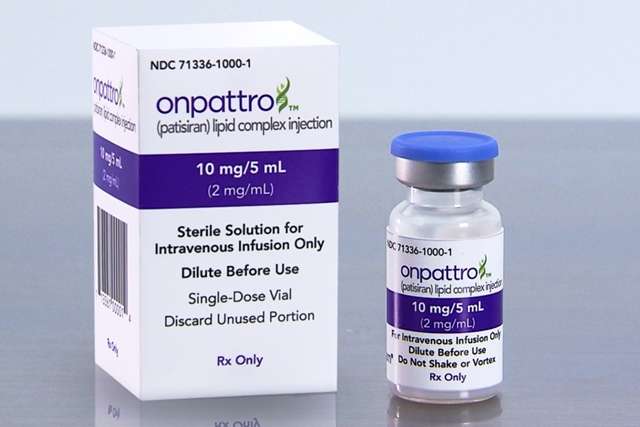
Another key approval noted in the report is for Onpattro, made by Alnylam Pharmaceuticals Inc. in Cambridge, Massachusetts. Onpattro treats peripheral nerve disease affecting nerves in the body other than the brain or spinal cord. The disease results from accumulations of amyloid protein fibers on peripheral nerves in tissues and organs, leading to loss of sensation or pain in the limbs, hands, and feet. The condition also can cause life-threatening disruptions of heart, kidney, eye, and gastrointestinal tract functions. Onpattro works by interfering with ribonucleic acid, or RNA, providing instructions from the genes to produce the errant proteins, with small interfering RNAs carried by lipid, or natural oil nanoscale particles, infused directly to the liver where the proteins are made.
The report credits FDA for creating a regulatory environment that supports development of precision medicines. In addition to the guidance published on treatments targeting biomarkers, the agency also issued a document on next-generation sequencing for diagnostics designed to test for mutations responsible for diseases that precision medicines could serve. In addition, FDA recognized a public database of genomics and diseases, the ClinGen Expert Curated Human Genetic Data resource, to provide standard benchmarks for evaluating diagnostics using genetic data to detect disease conditions based on variations in a person’s DNA.
However, the report also slams a Trump Administration policy begun in August 2018 to require “step therapy” for patients covered by Medicare Advantage. Under step therapy, doctors are required to first try less expensive standard treatments before more expensive therapies, even personalized medicines addressing the patient’s own molecular make-up.
“Driving treatment decisions by cost considerations rather than determining what treatment would work best for an individual patient,” notes the report, “fundamentally conflicts with personalized medicine, and in many cases will increase downstream costs brought on by continued progression of disease and more adverse side effects.”
More from Science & Enterprise:
- Precision Lung Cancer Trial Expands Scope
- Fitbit Devices Providing Precision Medicine Data
- FDA Identifies Database to Evaluate Precision Diagnostics
- Project to Catalog Precision Lung Cancer Immunotherapies
- Genome Centers Named for Precision Medicine Program
* * *

 RSS - Posts
RSS - Posts
[…] FDA Approves 25 Precision Meds in 2018 […]