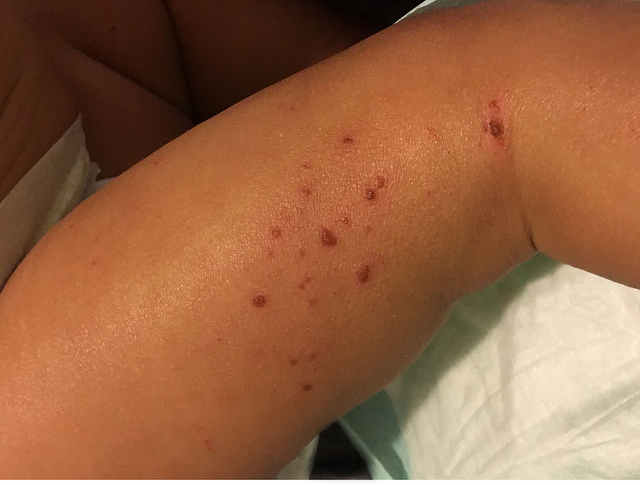
Child with atopic dermatitis or eczema (National Institute of Allergy and Infectious Diseases, Flickr)
6 Mar. 2019. A clinical trial is underway testing a form of bacteria sprayed on the skin that oxidize ammonia as a treatment for atopic dermatitis, or eczema, in children and teens. AOBiome Therapeutics Inc. in Cambridge, Massachusetts, the therapy’s developers, says today the study team treated its first patient.
Atopic dermatitis is a disorder that turns the skin red and itchy. The condition occurs most often in children, but can happen at any age, as a chronic condition that also flares up periodically. At present there is no cure for atopic dermatitis, with treatments like medicated creams and ointments designed to relieve the itching. The condition, while not considered dangerous, can lead to infections and disrupted sleep. AOBiome cites data showing 9.6 million children, or 13 percent of those under the age of 18 in the U.S., have atopic dermatitis, with nearly half of those with the condition later developing asthma or seasonal allergies.
AOBiome develops skin care products and therapies for several disorders that reintroduce ammonia oxidizing bacteria eliminated from the skin microbiome through modern hygienic practices. Ammonia-oxidizing bacteria, says the company, convert ammonia and urea from perspiration to nitrite and nitric oxide. Nitrite helps control the growth of other microbes, including pathogens, while nitric oxide is a signaling molecule that helps regulate inflammation.
The company’s lead product, code-named B244 is a strain of the bacterium Nitrosomonas eutropha formulated for different conditions and potency to treat various forms of inflammation on the skin and elsewhere. As reported in Science & Enterprise, formulations of B244 are being tested by AOBiome, or AOB to treat acne vulgaris and as a nasal spray for seasonal allergies. The company is already testing B244 in a mid-stage trial among adults with atopic dermatitis.
The new clinical trial is enrolling 36 children and teens age 2 to 17 with mild to moderate atopic dermatitis, with the entire sample divided equally into 3 age groups: 2 to 5, 6 to 11, and 12 to 17 years. The state of participants’ disease condition will be assessed at the outset, then they or their parents will be asked to spray on B244 to affected skin areas twice a day for 28 days. Participants will be assessed at the end of 7, 14, 21, and 28 days, with the study team looking primarily for adverse events and general physical condition during the period. In addition, researchers will look for changes in participants’ skin areas with atopic dermatitis, as well as the individuals’ own assessments of their condition.
“Our goal,” says Judith Ng Cashin, AOBiome’s chief medical officer in a company statement, “is to alleviate both the symptoms that are associated with atopic dermatitis and to utilize AOB’s nitric oxide-mediated anti-inflammatory abilities coupled with its capability to reduce levels of pathogenic bacteria as a dual-modality approach to treatment.” Cashin adds, “Current therapies for atopic dermatitis can cause side effects such as stinging, burning, and thinning of skin, especially in pediatric patients.”
The company’s use of B244 for treating atopic dermatitis was aided in January with awarding of a U.S. patent for AOBiome’s technology applied to this disease. AOBiome expects to release initial results from this trial in the second half of 2019.
More from Science & Enterprise:
- Engineered Microbe Blocks Ammonia Blood Poisoning
- Biotech, Institute Partner on Gut Microbe Links to Parkinson’s
- Soil Microbes Yield Rich Antibiotic Sources
- Natural, Engineered Microbes Shown to Control Cholera
- Genentech Gains Microbe Drug Discovery Tech in $1B Deal
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.