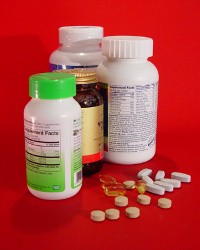
U.S. Pharmacopeial Convention, a pharmaceutical industry standards body in Rockville, Maryland, unveiled its Medicines Quality Database (MQDB) that provides information on the quality of medicines in Africa, South America and Southeast Asia. The database, funded by the U.S. Agency for International Development, has more than 8,700 records of tested samples collected in Ghana, Laos, Vietnam, Cambodia, the Philippines, Thailand, Peru, Guyana and Colombia.
MQDB aims to help health authorities and the general public identify poor-quality medicines circulating in several regions of the world. The database is part of a larger program to provide technical assistance to strengthen the quality assurance of medicines, particularly those intended to treat malaria, HIV/AIDS, and tuberculosis.
Data provided on the tested samples include the product name, manufacturer name, therapeutic indication, active pharmaceutical ingredient, dosage form, and batch/lot number. The records also give the results of authenticity tests, and counterfeit or substandard status of the drug. The data indicate the location and date of the samples, as well as data on the facilities where the samples were taken.
Information in the MQDB is collected from health authorities responsible for testing the quality of medicine samples in a given country. Data on tested samples are included in the MQDB only after verification for accuracy.
Read more: Texting Service To Fight Counterfeit Malaria Drugs
* * *

 RSS - Posts
RSS - Posts
[…] Read more: Database to Track Substandard, Counterfeit Medicines […]
Dear Alan,
I was wondering on a similar note,, The latest innovations in asset tracking for business owners are GPS monitoring systems. They can be used to locate vehicles, equipment and other valuable assets around the country or around the world. Here’s a brief look at how it works.
Thanks
Thanks Sonny for your comment and readership of Science Business. Supply chain integrity is a concern of a lot of industries. There’s no reason why the processes developed for monitoring the drug supply chain could not be applied to other industries. – AK