Engineers from the U.S. and China devised a foam substance made from graphene that is more sensitive in detecting potentially dangerous and explosive chemicals than current technologies. The researchers from Rensselaer Polytechnic Institute in Troy, New York and the Chinese Academy of Sciences in Shenyang published their research in the journal Scientific Reports.
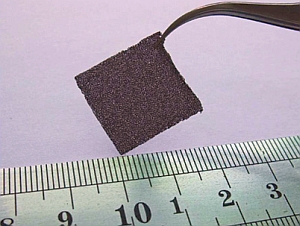
The graphene-based material, with potential applications in law enforcement, defense, and industry, is made from continuous graphene nano-scale sheets that grow into a solid foam structure about the size of a postage stamp and the thickness of felt (one nanometer equals one billionth of a meter). The authors say their process for making the material overcomes earlier barriers that prevented nanostructure-based gas detectors from reaching the marketplace.
Those earlier processes required building and manipulating individual nanostructures, which call for expensive microscopic and lithographic technologies to apply expensive gold contacts. In this project, the Shenyang team grew graphene on a macroscopic — observable with the naked eye — structure of nickel foam. Removing the nickel foam left a free-standing network of foam-like graphene.
Graphene is a one-atom-thick sheet of carbon atoms arranged like a nanoscale chicken-wire fence. The walls of the foam-like graphene sensor are made of continuous graphene sheets with no physical breaks or connections between the sheets.
The Rensselaer researchers developed the material into a gas detector and tested it on two gases: ammonia and nitrogen dioxide. The two gases adsorbed — attached a thin molecular layer of gas particles — to the graphene foam surface. This attachment of particles changed the surface chemistry and thus the electrical resistance of the graphene. Measuring this change in electrical resistance makes it possible for the sensor to detect different gases.
In the tests, the graphene foam detected ammonia at 1,000 parts-per-million in five to 10 minutes at room temperature and atmospheric pressure, with an accompanying change in the graphene’s electrical resistance at about 30 percent. Commercially available sensors made from polymers, require 10,000 parts-per-million of ammonia, and often a strong electrical power source and high temperatures, to undergo a 30 percent resistance change in five to 10 minutes. The authors say the graphene foam’s sensitivity is effective at detecting concentrations as low as 20 parts per million.
Ammonia is a common gas used in industrial and medical settings, but because it is also a toxic gas, systems using ammonia must be closely monitored for leaks. Ammonium nitrate is used in fertilizers, but also in many explosives. The chemical gradually decomposes and releases trace amounts of ammonia, thus ammonia detectors are often used to test for the presence of ammonium nitrate explosives.
With nitrogen dioxide, the graphene foam sensor detected concentrations at 100 parts-per-million by a 10 percent resistance change in five to 10 minutes at room temperature and atmospheric pressure, and could detect concentrations as low as 20 parts per million. The sensor demonstrated a sensitivity 10 times higher than commercially available conducting polymer sensors, which typically detect nitrogen dioxide at 1,000 part-per-million in the same time and with the same resistance chance at room temperature. Like current ammonia-detecting technology, today’s nitrogen dioxide sensors require strong power sources and high temperatures.
Nitrogen dioxide is emitted when nitrocellulose, an explosive, pulpy cotton-like substance also known as gun cotton, degrades, and thus is used as an indicator of explosives. Nitrogen dioxide is also a common pollutant found in combustion and auto emissions.
Engineering Professor Nikhil Koratkar, who led the Rensselaer team, calls the discovery, “the first practical nanostructure-based gas detector that’s viable for commercialization.” Koratkar adds that the graphene foam can be engineered to detect many different gases beyond ammonia and nitrogen dioxide.
Read more: Simple, Safer Process Developed to Synthesize Graphene
* * *

 RSS - Posts
RSS - Posts
Dear Sciencebusiness,
On a similar note,, Natural gas is one of the most popular sources of energy for homes and it is wildly used especially in well-developed countries that have laid out the proper infrastructure for it. Natural gas is distributed through gas pipes that usually run underground and into your house. And while it is a rare event, gas leaks do happen and can cause damage to people’s property or worse, could hurt those inhabiting the affected house.
Wishes
Thanks for your comment and for visiting Science Business. You make an excellent point about the need to detect natural gas leaks, such as methane, given their dangers to homes. In this case, the detector was aimed more at spotting gases emitted from explosive materials, such as ammonium nitrate — which was used in the bombing of a U.S. government building in Oklahoma City in 1995 — and nitrogen dioxide. But your idea would certainly have wider application. – AK