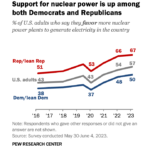
As the climate crisis focuses more attention on alternatives to fossil fuels, nuclear energy is gaining more popular support in the U.S. as one of those alternatives. . . . → Read More: Infographic – Nuclear Power Support Grows in U.S.
|
|||||

As the climate crisis focuses more attention on alternatives to fossil fuels, nuclear energy is gaining more popular support in the U.S. as one of those alternatives. . . . → Read More: Infographic – Nuclear Power Support Grows in U.S. 
The U.S. Food and Drug Administration issued new regulatory guidance for clinical trials of medical devices designed to prevent or treat opioid use disorder. . . . → Read More: FDA Issues Guidance for Opioid Therapy Device Trials 
A biotechnology company creating living tissue from a person’s stem cells says it received FDA clearance for a clinical trial of its replacement knee cartilage. . . . → Read More: Trial Cleared for Lab-Grown Replacement Knee Cartilage 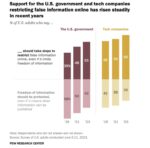
A recent survey shows majorities of American adults would like technology companies or the government to restrict the spread of false information online. . . . → Read More: Infographic – Most in U.S. Favor False Online Info Limits 
A treatment for HIV taken just once, using the genome-editing technology Crispr, is receiving fast-track designation from the U.S. Food and Drug Administration. . . . → Read More: One-Time Crispr HIV Therapy Given Fast-Track Status 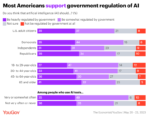
A recent survey shows a solid bipartisan majority of U.S. adults favors at least some government regulation of artificial intelligence, including people who say they use A.I. . . . → Read More: Infographic – U.S. Public Backs A.I. Regulation 
A review of insurance claims shows patients facing obstacles to coverage for their migraine therapies are also more likely to need emergency care for treatment. . . . → Read More: Insurance Barriers, Migraine Hospital Visits Linked 
A study of insurance claims records matching the design of randomized clinical trials shows claims data can in some cases resemble clinical trial results to make causal inferences. . . . → Read More: Real World Evidence Shown to Emulate Some Clinical Trials 
A biotechnology industry group calls Friday’s ruling in a Federal district court to suspend approval of the drug mifepristone “an assault on science.” . . . → Read More: Biotech Group Blasts Reproductive Drug Ruling 
A developer of treatments for eye diseases says it received clearance from FDA to begin a clinical trial of a sustained-release implant therapy for glaucoma. . . . → Read More: Trial Okayed for Glaucoma Treatment Implant |
|||||
|
Copyright © 2024 Technology News and Literature - All Rights Reserved Powered by WordPress & Atahualpa |
|||||