Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on September 9th, 2023% 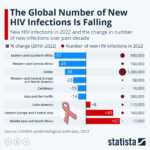
The number of new infections from the human immunodeficiency virus or HIV declined worldwide last year and over the past decade, with the sharpest declines in sub-Saharan Africa. . . . → Read More: Infographic – HIV Infections Fall in Africa. Rise in Mideast, Europe
By Alan, on July 20th, 2023% 
A treatment for HIV taken just once, using the genome-editing technology Crispr, is receiving fast-track designation from the U.S. Food and Drug Administration. . . . → Read More: One-Time Crispr HIV Therapy Given Fast-Track Status
By Alan, on December 3rd, 2022% 
The data show falling numbers of young men and women in sub-Saharan Africa with new HIV infections since 2010, but the percentage of infected women and girls also rising over that time. . . . → Read More: Infographic – Higher HIV Burden for Women, Girls in Africa
By Alan, on October 26th, 2022% 
Results from a clinical trial show an experimental one-time antibody therapy, even at low doses, lowers levels of HIV virus in people living with the disease. . . . → Read More: Trial Shows Antibody Neutralizes, Reduces HIV Virus
By Alan, on May 12th, 2022% 
The medical products company Roche is partnering with the Global Fund to develop systems and processes for disease diagnostics in low-resource regions. . . . → Read More: Roche, Global Fund to Build Local Diagnostic Processes
By Alan, on January 27th, 2022% 
A clinical trial is underway assessing a therapy for HIV infections that aims to eradicate the virus with one dose of the gene-editing technique Crispr. . . . → Read More: Clinical Trial Underway for One-Time Crispr HIV Treatment
By Alan, on September 15th, 2021% 
A company developing a one-time treatment for HIV infections using the gene-editing technique Crispr says it received FDA clearance to begin a clinical trial. . . . → Read More: FDA Okays One-Time HIV Treatment Clinical Trial
By Alan, on August 9th, 2021% 
A biotechnology company received a foundation grant for an implanted device to prevent HIV infection and unwanted pregnancy in women. . . . → Read More: Gates Grant Funds HIV-Birth Control Implant
By Alan, on February 1st, 2021% 
A biotechnology company is adapting and licensing its immunotherapy process, originally designed for cancer, as a cure for HIV infections. . . . → Read More: Cancer Immunotherapy Adapted for HIV
By Alan, on January 22nd, 2021% 
The Food and Drug Administration approved a drug regimen given once a month to control HIV infections, to replace daily antiretroviral medications. . . . → Read More: Monthly HIV Drug Combo Approved by FDA
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|







 RSS - Posts
RSS - Posts