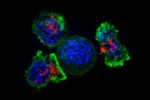
The developer of an experimental immunotherapy delivering nanoscale payloads to treat pancreatic cancer says the treatment received fast-track status from the Food and Drug Administration. . . . → Read More: Nanotech Pancreatic Cancer Immunotherapy Gains FDA Fast Track


 RSS - Posts
RSS - Posts