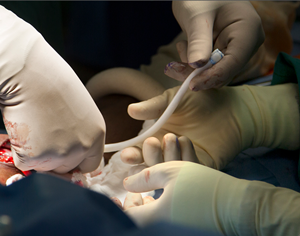
Yesterday, a kidney dialysis patient at Duke University Hospital in Durham, North Carolina was the first in the U.S. to receive a new bioengineered blood vessel developed by a Duke University spin-off company. The patient, a 62 year-old man from Danville, Virginia with kidney failure received in his arm the engineered blood vessel made with a process developed by Humacyte Inc., also in Durham.
A new vein is often required by patients with kidney disease needing dialysis, who receive a graft to connect an artery to a vein to speed the flow of blood during treatments. Current options for this procedure all have drawbacks: Synthetic veins are prone to blood clots, harvesting other veins for transplants requires separate surgery with higher risks of infection. Veins made from a person’s own cells takes an inordinate amount of time to grow and are not amenable to advance production.
The Humacyte technology for generating bioengineered veins is the result of a research collaboration between Duke medical professor Jeffrey Lawson — who performed the implantation — and former Duke postdoctoral fellow Laura Niklason, who went on (with colleagues) in 2005 to start Humacyte. Niklason, now at Yale University, and Lawson first tried to create new bioengineered veins from a patient’s own cells, later shifting to a process that still creates a usable engineered vein, but instead with compatible and neutral biomaterials.
The process starts with a flexible biodegradable mesh serving as a scaffold for the vein that can be shaped into a tube. The scaffold is then seeded with donated human smooth muscle cells that grow in a medium of amino acids and nutrients. The growing muscle cells are subjected to a pulsating flow resembling a heartbeat rhythm that strengthens the engineered tissue and results in physical properties resembling natural vascular tissue.
The tubular seeded scaffold is then washed in a solution that rinses out cellular properties, leaving a biocompatible collagen tube that does not trigger an immune response from the implanted patient. The process for creating this allogeneic — same species, different donor — replacement tissue takes about two months.
These engineered veins, says Humacyte, can be created in advance, then stored for use later on. Preclinical tests with baboons show the engineered veins perform better than those made from synthetic or animal-based materials. The patient implanted yesterday is part of an early-stage safety trial of the engineered vein with 20 dialysis patients. A similar clinical trial is underway as well in Poland.
Data from 2006 suggest more than 350,000 people in the U.S. are receiving dialysis treatments. Niklason and Lawson believe the technique can also be applied to the nearly 400,000 people in the U.S. who have heart bypass surgery, as well as patients with blocked blood vessels in their limbs. Humacyte says it has a pilot manufacturing facility meeting current good management practices for pharmaceuticals, and expects to broaden its pilot-scale facility during 2013.
Read more:
- Silk, Cellulose Provide Useful Scaffold to Repair Cartilage
- University, Biotech Develop Heart Tissue Repair Patch
- Synthetic Tissue Created with Water, Lipids, 3-D Printing
- Anatomical Models 3-D Printed from Tomography Scans
- Retinal Cells Produced Without Animal Matter from Stem Cells
* * *

 RSS - Posts
RSS - Posts
[…] First Bioengineered Vein Implanted for U.S. Dialysis Patient […]