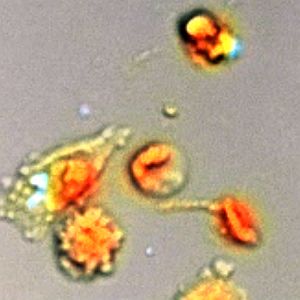
Enhanced image of melanoma cancer cells, dyed red, attacked by T cells, dyed blue, when activated by a melanoma specific ImmTAC (Immunocore Ltd.)
MedImmune in Gaithersburg, Maryland, the biologics division of pharmaceutical company AstraZeneca, and Immunocore Ltd. in Oxford, U.K. will develop cancer treatments that harness the body’s immune system, based on Immunocore’s technology. The deal will pay Immunocore, for each therapy program licensed by MedImmune, $20 million initially and another $300 million in milestone payments, as well as royalties on sales from products developed by MedImmune under the agreement.
Immunocore says its technology creates engineered molecules called Immune Mobilizing Monoclonal T-Cell Receptor Against Cancer or ImmTACs, which find and control disease cells that would normally escape recognition by the immune system. T cell receptors are viral fragments appearing on the surface of T lymphocytes, the white blood cells used by the immune system to fight invading viruses. These receptors attract the antigens that bind with antibodies, the molecules that do the fighting.
The company says ImmTACs are monoclonal or highly targeted T-cell receptors that control diseased cells more effectively than monoclonal antibodies, the usual method employed with cancer immunotherapies. The engineered T-cell receptors, says the company, enable the killing of cancer cells aimed at proteins on the surface of the cell — like monoclonal antibodies — but unlike monoclonal antibodies, can also find targets inside the cells, including proteins secreted by the cells. The precise targeting makes it possible to attack only the cancer cells, not healthy cells.
Immunocore, founded in 2008, is a spin-off company from Avidex, a biotechnology enterprise itself spun-off from Oxford University in 1999. Avidex was founded by Oxford immunologist Bent Jakobsen, who is now Immunocore’s chief scientist. The company has one immunotherapy candidate, code-named IMCgp100, in clinical trials as a treatment for malignant melanoma.
MedImmune develops biologic therapies and vaccines, with FDA-approved flu vaccines and therapies for lower respiratory tract disease in children caused by respiratory syncytial virus. The company says it has 120 biologics in its development pipeline covering respiratory, inflammation and autoimmunity, cardiovascular, metabolic diseases, cancer, neuroscience, and infectious diseases.
Read more:
- Early Trial Tests Stem Cell Immunotherapy for Liver Cancer
- Start-Up Licenses UMass Cancer Immunotherapy Technology
- Immunotherapy Shown Effective in Brain Tumor Animal Test
- Roche, Biotech Ink Cancer Vaccine/Immunotherapy Deal
- Bayer, Compugen to Partner on Cancer Immunotherapies
Hat tip: FirstWord Pharma
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.