Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on August 8th, 2023  (7089643, Pixabay. https://pixabay.com/vectors/women-silhouettes-girl-woman-4918254/) 8 Aug. 2023. A women’s health biotechnology company is partnering with a Brazilian drug maker to evaluate a precision target and treatment for ovarian health disorders. The collaboration gives Celmatix Inc. in New York an option to license the therapy discovered by Aché Laboratórios in Guarulhos, Brazil, but no financial details of the agreement were disclosed.
Celmatix discovers and develops through preclinical stages treatments for ovarian health conditions that exhibit specific genomic properties. The company’s technology is based on research in reproductive health by its founder and CEO Priaye Yurttas Beim while a postdoctoral researcher at University of Cambridge in the U.K. Celmatix says it maintains women’s health databases on genomics, medical histories, and lifestyle. And, says the company, those data collections are annotated with new research findings analyzed by natural language processing and machine learning algorithms.
The lead program at Celmatix is a treatment candidate for polycystic ovary syndrome or PCOS, a disorder marked by excess androgens or male sex hormones and formation of cysts in the ovaries. PCOS often results in infertility, missed or irregular periods, excess body hair, weight gain, and thinning hair. Celmatix cites data showing PCOS is a leading cause of infertility affecting 15 percent of women in reproductive age, and can affect a range of other health conditions. PCOS treatments so far address symptoms, not the underlying causes.
Melatonin signaling outside the central nervous system
The Celmatix PCOS therapy candidate is designed to stimulate melatonin receptor proteins that bind to melatonin, a hormone produced by the pineal gland in the brain that regulates the body’s circadian rhythm. The company says its research indicates melatonin signaling also occurs outside the central nervous system or CNS, extending to peripheral tissue including the ovaries. Celmatix says its treatment candidate targets rare mutations in melatonin receptor genes in women associated with the disease, thus addressing its underlying causes. The company says its PCOS therapy is designed to activate only in ovarian tissue to minimize adverse effects, such as drowsiness.
“We know there is a compelling link,” says Beim in a Celmatix statement released through BusinessWire, “between melatonin signaling outside of the CNS and endocrine, metabolic, and reproductive traits in women with PCOS. However, melatonin and existing selective melatonin receptor agonists are not viable therapeutics for ovarian health conditions because they have potent CNS-activity that creates significant side effects, including nausea and drowsiness.”
Aché Laboratórios says it independently discovered a melatonin receptor agonist as a treatment candidate for PCOS. The agreement calls for Aché and Celmatix to jointly assess the Aché melatonin receptor agonist in preclinical animal tests. If the studies are successful, the companies will negotiate a separate license and development agreement. Aché says it adds new markets through licensing agreements outside Brazil, now reaching 17 countries.
“By focusing on melatonin receptor action outside of the CNS,” adds Beim, “through this joint development and licensing option agreement with Aché, we are one important step closer to being able to offer relief to the millions of women with a range of ovarian health conditions, including PCOS.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on August 7th, 2023  Aedes aegypti mosquito biting a human (U.S. Dept of Agriculture) 7 Aug. 2023. Initial findings from a large clinical trial testing a vaccine against the chikungunya virus show the vaccine generates protective antibodies in the vast majority of recipients. The results were released by biopharmaceutical company Bavarian Nordic A/S in Copenhagen, Denmark, developer of the candidate vaccine code-named CHIKV VLP, and are not yet peer-reviewed.
Chikungunya is a viral disease spread by mosquitoes, causing fever and severe joint pain, and often joint swelling, headache, nausea, fatigue, and rash. Symptoms in most people last a few days, but for others can continue for weeks and months, and become debilitating. Because chikungunya symptoms are similar to Zika and dengue, misdiagnoses are common, and the condition is often underreported. The disease was first diagnosed in Africa and Asia, where Aedes aegypti and Aedes albopictus mosquitoes reside, but has since spread to the Americas, including the U.S. As yet, there are no approved treatments nor vaccines for chikungunya.
Bavarian Nordic is an established vaccine developer and manufacturer with several products approved and on the market for encephalitis, smallpox and monkeypox, rabies, cholera, and typhoid. Of its vaccines in development, chikungunya and SARS-CoV-2 are in late-stage clinical trials. The company’s technology is derived from modified vaccinia Ankara, a weakened and benign form of smallpox that does not replicate in recipients. Bavarian Nordic says the technology produces vaccines that generate both antibodies as well as cellular immune responses. The company’s CHIKV VLP candidate vaccine delivers virus-like particles on an aluminum hydroxide adjuvant designed to induce immune responses in recipients.
Protective levels of neutralizing antibodies
Bavarian Northern is testing CHIKV VLP in two late-stage clinical trials among healthy participants, one study with people age 65 and over and the other with younger adults and adolescents, age 12 to 64. In the latter trial with results reported yesterday, the study team enrolled 3,254 participants across the U.S. Participants were randomly assigned to receive a single dose of CHIKV VLP vaccine or a placebo, with individuals first tracked for 22 days, then six months. Researchers looked for anti-chikungunya antibody concentrations in blood samples after those intervals, as well as adverse effects.
Findings reported by Bavarian Northern show CHIKV VLP induces protective levels of neutralizing antibodies against chikungunya viruses in 98 percent of recipients after 22 days, with the percentage of participants with protective levels of antibodies dropping off somewhat to 86 percent after six months. The company says the vaccine is well tolerated among adolescents and adults up to age 64 with adverse effects rated mild or moderate, but no efficacy nor adverse effects results from the placebo group are reported.
In the second late-stage clinical trial, among 413 healthy adults age 65 and over and reported in June 2023, Bavarian Northern says 82 percent reported protective concentrations of antibodies after 15 days, with that percentage growing to 87 percent by 22 days. The company says the vaccine was well tolerated among recipients with rates of adverse effects similar for vaccine and placebo recipients. Six-month follow-up data, says the company, are still being collected.
Paul Chaplin, president and CEO of Bavarian Nordic, says in a company statement, “Our focus remains to finalize the studies and prepare for regulatory submissions next year.” Chaplin adds, “Chikungunya can often result in a severe and incapacitating disease that affects large parts of the world, and with international travel on the rise again, our CHIKV vaccine offers a significant opportunity to address this large unmet medical need.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on August 5th, 2023 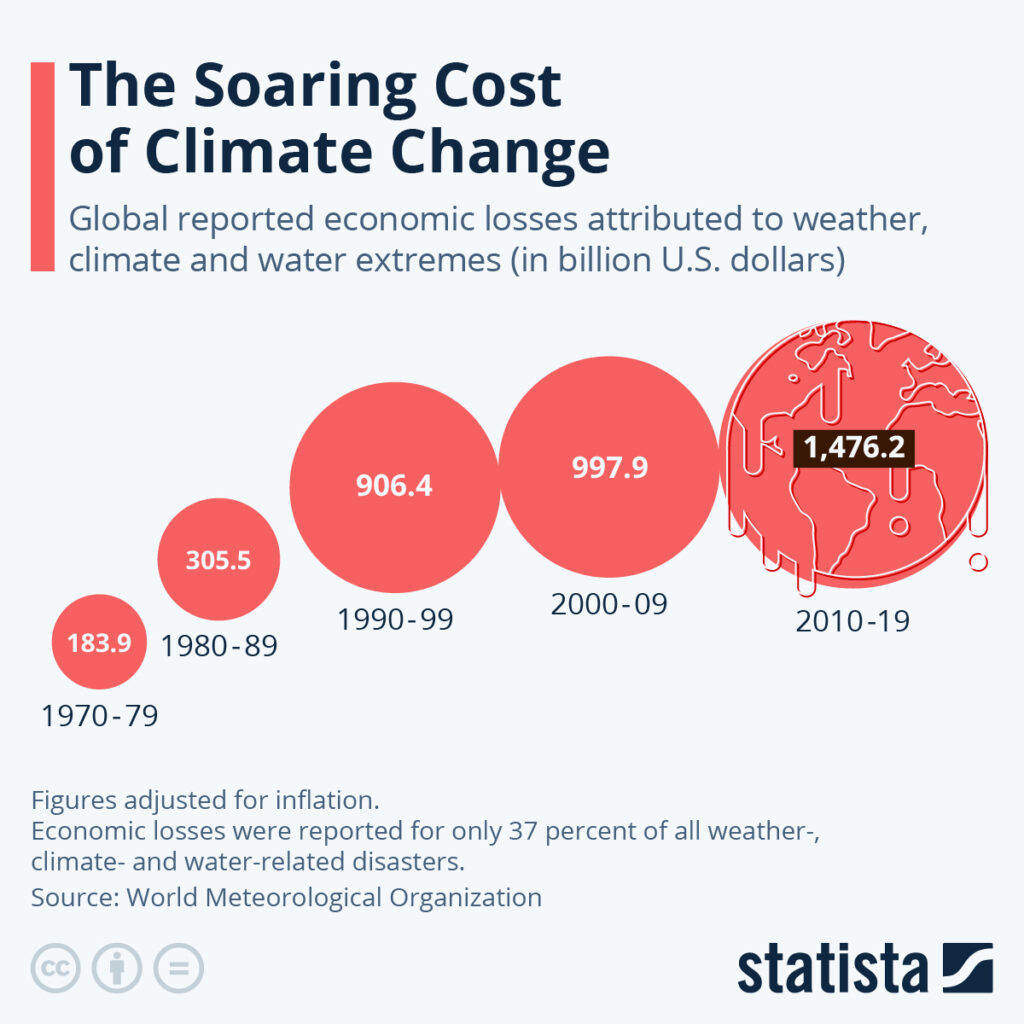 Click on image for full-size view (Statista) 5 Aug. 2023. Few people in the world need a reminder that the climate is changing quickly, and for the worse. Wildfires, extreme storms, and more flooding are becoming worldwide phenomena of a deteriorating climate from growing greenhouse gas emissions. The business research company Statista posted a chart last month illustrating the growing economic price tag of that climate crisis.
Statista drew the data from a report issued in May from the World Meteorological Organization seeking to put a dollar value on losses from the climate crisis, at least through 2019. WMO tracked natural disasters related to weather, climate, or water through this 50-year period, and calculated their reported numbers of deaths and economic costs. In the last three decades, according to the analysis, the economic burden jumped markedly from before, rising to nearly $1.5 trillion worldwide in the years 2010 to 2019.
The WMO report indicates extreme storms and flooding account for the bulk of economic losses from the climate, weather, and water-related disasters since 2010, but the proportion of losses from wildfires also grew as a part of the total since that time. The largest cause of deaths from these causes since 2010, some 184,000, is extreme temperatures. And according to WMO, the financial numbers may be gross under-estimates. The agency says costs of natural disasters from the climate crisis are reported mainly from developed countries, with 63 percent of losses going unreported.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on August 4th, 2023  (Gerd Altmann, Pixabay. https://pixabay.com/illustrations/digitization-particles-smartphone-7261158/) 4 Aug. 2023. A new company with a process using artificial intelligence and robotics to speed development of sustained release drugs is raising $4 million in seed funds. Persist AI, founded last year in Woodland, California, says its technology can cut the amount of time in half for developing drugs, designed for release in the body over longer periods to treat chronic diseases.
Persist AI says drug makers typically need up to five years to develop drugs that release their contents over extended periods, to treat chronic disorders such as diabetes and cancer. The vehicle for these longer-acting compounds is the polymer poly lactic-co-glycolic acid or PLGA, a biocompatible and biodegradable material with desirable physical and chemical properties for sustained release medications. PLGA can be formulated for small molecule or low molecular weight drug delivery and biologics, such as proteins, peptides, and nucleic acids. In addition, some of its physical properties can be tuned or adjusted to optimize dosage or release interval.
For extended release drugs, PLGA is formulated into microscale spheres that allow for processing into the needed drug delivery properties, such as for use with inhalers or delivery into the eye. Persist AI says this microsphere formulation stage often acts as the bottleneck in developing long-acting injectable medications. The company says its analytics use A.I. models to screen up to 500 different microsphere formulations each month. These models predict activity of different formulations in the body, and determine a minimum number of injections needed by patients. And, says Persist AI, it uses robotics to accelerate testing of new formulations.
Y Combinator participant earlier in 2023
“We complete microsphere pre-formulation for clients in weeks, not years,” says co-founder and CEO Karthik Raman in a Persist AI statement released through Cision. The company’s development so far also followed a fast track. Persist AI took part in the Y Combinator accelerator earlier this year, a three-month program for technology company founders with mentorship and training in business start-up, marketing, team building, and product development. Participants prepare for pitching their company to investors and media, as well as receive a pre-seed investment of $500,000.
Persist AI says today it raised $4 million in seed funds, led by technology investor 2048 Ventures in New York. Taking part in the round are Innospark Ventures, Fellows Fund, Pioneer Fund, and YCombinator. 2048 Ventures typically invests in pre-seed, seed-, and series A or first venture rounds.
“Drug formulation has long been overlooked despite being a crucial part of drug development,” notes Alex Iskold, managing partner of 2048 Ventures. “Persist’s technology allows them to rapidly screen formulations, develop AI models that optimize the formulations, and then scale up formulations for human clinical use.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on August 3rd, 2023  Scanning electron microscope image of a breast cancer cell. (Bruce Wetzel and Harry Schaefer, National Cancer Institute, NIH, Flickr https://flic.kr/p/K4F3ZT) 3 Aug. 2023. A clinical trial assessing a synthetic antibody to generate an immediate response and also generate a more durable treatment for solid tumor cancers, advanced to a mid-stage study. Bolt Biotherapeutics Inc. in Redwood City, California,, developer of the experimental cancer treatment code-named BDC-1001, says the first patients in the new stage of the trial received doses of the therapy.
Bolt Biotherapeutics creates cancer treatments that invoke the immune system, but in a more complex mechanism than most other immunotherapies. The company’s process called immune-stimulating antibody conjugate or ISAC combines or conjugates synthetic highly-targeted monoclonal antibodies with drugs designed to activate the innate or general immune system. This approach, says Bolt Bio, makes it possible to address solid tumor cancers that would be otherwise difficult to treat, and in patients where cancer does not respond to conventional treatments or recurs. And, says the company, the dual-action mechanism helps generate a longer-term response to tumors.
In this case, BDC-1001 targets cancers expressing human epidermal growth factor receptor 2 or HER2-positive proteins, often associated with breast cancer, but also found in other tumors. Bolt Bio says BDC-1001 contains a biologic similar to the monoclonal antibody trastuzumab, combined with the company’s own version of toll-like receptor proteins 7 and 8, or TLR7/8. Trastuzumab binds to and blocks HER2 activity in tumors and invokes a localized attack on tumor cells, while TLR7/8 proteins generate T-cells from the innate immune system to provide a more durable response.
29 percent clinical response to treatment
Bolt Biotherapeutics is enrolling in the clinical trial 390 individuals in the U.S., Spain, and South Korea diagnosed with breast, colorectal, uterine, and stomach cancer expressing HER2-positive proteins. The study has four parts, with the first two parts making up an early-stage trial evaluating safety, tolerability, and maximum dose for BDC-1001. However, the study has no placebo group for control purposes.
In June 2023, Bolt Bio reported initial findings from the trial at a meeting of American Society of Clinical Oncology. Results show an optimal dose of 20 milligrams per kilogram of weight, infused once every two weeks, with most adverse effects rated mild or moderate at the infusion site. In addition, 29 percent of participants show a clinical response to BDC-1001, either on its own or combined with nivolumab, a checkpoint-inhibitor immunotherapy.
The study now moves into its third part, a mid-stage trial assessing BDC-1001 as a treatment on its own for HER2-positive breast, colorectal, uterine, and stomach cancer. The study team is tracking participants for two years, again looking for adverse effects from the treatments and immune-related toxicities, but also chemical effects in the body, as well as clinical responses, duration of response, and progression-free survival time. A fourth part of the trial is scheduled to follow, evaluating BDC-1001 taken with nivolumab.
“Despite considerable advances in anti-cancer therapy,” says Bolt Biotherapeutics chief medical officer Edith Perez in a company statement, “HER2-positive tumors remain difficult to treat, and new therapeutic options are urgently needed. Our ISAC platform brings a novel mechanism with the potential to address refractory and recurrent disease to the treatment of HER2+ cancers and BDC-1001 has demonstrated promise.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on August 2nd, 2023  (Colin Behrens, Pixabay. https://pixabay.com/illustrations/hand-molecule-chemistry-science-898232/) 2 Aug. 2023. A company, spun-off from academic labs that designs and produces new chemicals with artificial intelligence and robotics, is raising $42.7 million in its first venture funding round. Chemify, in Glasgow, Scotland, began operations last year, commercializing research by University of Glasgow chemistry professor and company founder Lee Cronin.
Cronin’s digital chemistry group at University of Glasgow studies the digitization of chemistry for discovery of molecules with algorithms and robotics to speed identification of new chemical compounds by overcoming physical limitations imposed by human intervention. The lab also studies the intersection of chemistry and computer science, adapting chemical models for electronics design, as well as integrating programming logic into chemical analysis. Another Cronin lab team investigates 3-D printing to create small custom chemical reactors for producing personalized drugs on demand.
Chemify seeks to bring these integrations of chemistry and digital technology to the marketplace. The company offers development of new molecules with A.I. algorithms and what it calls a chemical programing language that enables design of compounds meeting specified functional properties. Chemify says its robotics facilities make possible rapid design-make-test-analyze cycles, as well as parallel testing and rapid scale-up for pilot plant chemical synthesis. The company says its technology can speed development of new molecules for medicines, agriculture, materials, and green energy.
A.I. in biology, energy, and chemistry
“It has long been our dream to digitize chemistry,” says Cronin, also Chemify’s CEO in a company statement released through BusinessWire adding, “Chemify is building a company that can design, make, and discover complex molecules on demand using digital blueprints faster, more efficiently, and safely than is currently possible. Our mission is to deliver better molecules for pharmaceutical and industrial partners in a fraction of the time and cost currently required.”
Chemify is raising £33.6 million ($US 42.7 million) in its first venture funding round, led by technology investor Triatomic Capital in New York. Triatomic funds start-ups advancing A.I. and engineering applications in biology, energy, chemistry, and designed materials, as well as advances in A.I., robotics, and blockchain. Taking part in the round are current investor BlueYard Capital and new investors Horizon Ventures, Rocketship Ventures, Possible Ventures, Alix Ventures, Eos, and the U.K. Government Innovation Accelerators program.
Start-ups working at the intersection of chemistry, biotechnology, and engineering appear to be attracting venture investor interest in recent months. Since April 2023, Science & Enterprise has reported on several new companies in this space raising at least $5 million in seed or first venture rounds: Carbonwave developing biomaterials from Sargassum seaweed, Initial Therapeutics designing small molecule therapies that alter the the synthesis of proteins in cells, Hopewell Therapeutics developing nanoscale lipid particles for delivering genomic therapies to cells and tissue, and Camena Bioscience producing synthetic genes with greater accuracy for research and clinical applications.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on August 1st, 2023  Flowering Nicotiana benthamiana plant (Charles Andres, Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Nicotiana_benthamiana_flower.jpg) 1 Aug. 2023. The developer of a biologic drug-making process with engineered plant crops is collaborating with a vertical farming company to create a network of biologics production facilities. KBio, a subsidiary of British American Tobacco or BAT founded last year in Owensboro, Kentucky, is partnering with Zero Farms, a company in Italy seeking to make vertical farming more modular, scalable, economically viable, and sustainable.
KBio was formed to advance research by BAT in biotechnology, particularly production of synthetic antibodies and other biologic drugs. That research centered on harnessing properties of plants in the tobacco family to quickly and economically produce monoclonal antibodies, highly targeted synthetic proteins for vaccines and therapeutics. KBio says its process identifies and clones genes coding for specific monoclonal antibodies or other proteins to genetically engineer bacteria that infiltrate plants.
Plant species are submerged under vacuum pressure with the engineered bacteria to speed infiltration, where the plants incubate the bacteria for expressing the designed proteins contained in plant cells and tissue. The infected plants, says the company, are then exposed to tobacco mosaic viruses, normally a tobacco plant pest, but in this case used to extract monoclonal antibodies or other proteins from plant material. KBio says the protein outputs from plants are purified and subjected to rigorous testing required by Good Manufacturing Practices or GMP quality standards for drug manufacturing.
BAT researchers demonstrated this process in a paper published in June 2021 the journal Methods in Enzymology. The BAT team created and produced monoclonal antibodies meeting GMP standards in as little as 10 days, with Nicotiana benthamiana plants, a relative of tobacco native to Australia, and a model species for lab research in genetics and viral transmission. KBio says its process can produce GMP-quality monoclonal antibodies in about two weeks after infiltrating plants with engineered bacteria.
Modular design and extensive automation
Zero Farms is a five year-old enterprise near Venice that aims to make vertical farming, producing food crops and other plants in stacked greenhouse-like urban spaces, economically viable and sustainable. Up to now, says Zero Farms, vertical farming was considered an admirable goal, but lacking an economical means of producing plant crops quickly and inexpensively. In addition, says the company, most vertical farms use unique designs and are labor-intensive, making them difficult to attract financing for business growth.
To meet this challenge, Zero Farms says it started from scratch — or zero, the source of its name — to create growing centers with a basic modular design and extensive automation. The company’s software includes a cloud-based management package with algorithms derived from farmers, agronomists, and engineers that Zero Farms says makes it possible to economically operate a network of geographically distributed vertical farming facilities.
KBio and Zero Farms say they plan to establish a network of vertical farms for producing biologics and other bio-based materials with Nicotiana benthamiana plants. The companies say their proposed network could provide public health authorities with a means of quickly producing vaccines and therapeutics for responding to future pandemics. In addition, new modular growing facilities could be quickly deployed in emergencies.
“We plan to facilitate joint R&D activities with Zero,” says KBio CEO Patrick Doyle in a company statement released through Globe Newswire, “in the United States, U.K., and Italy that will involve the production of monoclonal antibodies, vaccines and various bio-industrial products with B2B applications in the food, nutraceutical, cosmetic, and pharmaceutical sectors.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on July 31st, 2023  (Bryan Brandenburg, Wikimedia Commons) 31 July 2023. A system using deep learning algorithms to analyze ultrasound video images is shown to detect a difficult-to diagnose type of heart failure with clips from one view of the heart. Researchers at the Mayo Clinic in Rochester, Minnesota and heart disease analytics company Ultromics Ltd. in Oxford, U.K. describe their findings in today’s issue of the journal JACC: Advances, and presented last month at a meeting of the American Society of Echocardiography.
Heart failure is a common and sometimes deadly condition, where the heart cannot pump enough blood to the rest of the body to support other organs. Centers for Disease Control and Prevention estimates some 6.2 million people in the U.S. have heart failure, with nearly 380,000 deaths attributed to the disease in 2018. People with coronary artery disease, high blood pressure, diabetes, and obesity are considered at the highest risk of heart failure.
One indicator of heart failure is reduced blood flow from the heart’s left ventricle, known as ejection fraction. The left ventricle pumps blood through the aorta providing oxygen for cells, tissue, and organs in the body. In some cases, the heart appears to be pumping normally, but the amount of blood pumped from the left ventricle is reduced, known as heart failure with preserved ejection fraction, or HFpEF. Because of its complexity, say the journal paper’s authors, HFpEF can be difficult to detect with conventional echocardiograms, an ultrasound technique for detecting heart disease in real time.
Ultromics is a six year-old company with a technology based on research at the University of Oxford lab of Paul Leeson, professor of cardiovascular medicine, who studies early indicators of heart disease. Among the lab’s work is applying artificial intelligence to improve heart disease diagnostics with echocardiograms. Ultromics collaborates with the Mayo Clinic on EchoGo Heart Failure, the company’s lead system for diagnosing HFpEF from echocardiogram images.
Combining image analysis and deep learning
Leeson co-founded Ultromics with former doctoral student Ross Upton in 2017, and serves as the company’s chief medical officer, while Upton is CEO. In Dec. 2022, Science & Enterprise reported on the EchoGo system gaining FDA authorization for marketing in the U.S.
In their paper, a team led by Mayo Clinic cardiologist Patricia Pellikkia, also director of the clinic’s echocardiography lab, describes development of the EchoGo model. The authors say EchoGo is based on algorithms in a convolutional neural network, a type of artificial intelligence that combines image analysis and deep learning. The algorithms discern components in images for training deep-learning analytics to reveal underlying patterns and relationships in those image components. As the algorithms encounter more images and components, the algorithms become better able to analyze new images.
In this case, EchoGo is trained with echocardiogram clips viewed from the apex or top of the heart chamber, capturing real-time video of all four heart chambers, called a transthoracic echocardiograph or TTE. The authors report training the model with TTE video from 2,971 individuals at the Mayo Clinic with HFpEF and 3,785 without the condition. In an independent validation with 1,284 individuals, divided about evenly between patients with and without HFpEF, EchoGo returned a true-positive sensitivity of 88 percent and true-negative specificity of 82 percent. Applying the model to more than 700 cases where conventional heart failure tests previously returned indeterminate results, EchoGo correctly identified 74 percent of individuals with HFpEF.
“HFpEF can be difficult to detect,” says Pellikkia in an Ultromics statement, “but left undetected and untreated, can result in hospitalization and mortality. As the first A.I. platform cleared to detect the condition, EchoGo Heart Failure can fill a significant unmet need.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on July 31st, 2023 – Sponsored content –
 (Cottonbro Studio, Pexels) 31 July 2023. In any organization, a strong team identity is essential for fostering unity, collaboration, and a sense of belonging among team members. When individuals identify with their team and feel like they are part of something greater than themselves, it can lead to improved productivity, increased job satisfaction, and higher levels of commitment. One often overlooked yet effective tool for building team identity is the humble lanyard, but its significance goes beyond its practical use. In this article, we’ll explore the concept of team identity, its benefits, and the role that items like lanyards can play in cultivating a cohesive and motivated team.
What is Team Identity?
Team identity refers to the shared sense of purpose, values, and belongingness among team members. It goes beyond individual roles and responsibilities and focuses on the collective identity of the team as a whole. A strong team identity fosters a culture of cooperation, mutual support, and trust, which are essential for achieving common goals.
The Benefits of a Strong Team Identity
Improved Communication:
Team members with a strong identity are more likely to communicate openly and honestly with one another. They feel comfortable sharing ideas and concerns, which leads to better problem-solving and decision-making processes.
Higher Morale and Motivation:
When team members feel connected to their group, they become more motivated to excel in their work. A strong team identity boosts morale, leading to increased enthusiasm and commitment.
Increased Productivity:
Teams with a clear identity are more efficient in their tasks. They know each other’s strengths and weaknesses and can assign responsibilities accordingly, resulting in higher productivity levels.
Greater Resilience:
A strong team identity fosters a sense of resilience during challenging times. Team members support and uplift each other, enabling them to overcome obstacles together.
Enhanced Innovation:
Team members who share a common identity are more likely to brainstorm and innovate collaboratively. The freedom to express unique perspectives within a cohesive framework encourages creativity.
The Role of Lanyards in Building Team Identity
Lanyards, typically worn around the neck, have long been associated with identifying individuals within an organization. However, their significance extends beyond their functional use of holding ID badges or access cards. Here’s how lanyards can contribute to building team identity:
Visual Representation:
Custom lanyards with team logos, colors, or slogans act as a visual representation of the team’s identity. Whenever team members wear these lanyards, they proudly showcase their affiliation with the team.
Sense of Belonging:
Lanyards create a sense of belonging among team members. When everyone wears the same lanyard, it symbolizes their membership and involvement in a particular team or project.
Ice Breaker:
Lanyards can serve as conversation starters, especially in larger organizations where employees from different departments might not know each other well. It facilitates networking and social interactions within the workplace.
Recognition and Appreciation:
Distributing customized lanyards to team members can be a way of recognizing their contributions and showing appreciation for their efforts. It communicates that they are valued members of the team.
Team-Building Events:
During team-building events or offsite retreats, wearing team lanyards can foster a sense of camaraderie and unity. It reinforces the team’s shared purpose and encourages participation in team-building activities.
Beyond Lanyards: Other Ways to Build Team Identity
While lanyards are an effective tool, team identity can be cultivated through various other means:
Team Charters:
Create a team charter that outlines the team’s mission, vision, values, and goals. This document serves as a guiding compass for the team and helps solidify its identity.
Regular Team Meetings:
Conduct regular team meetings to discuss progress, challenges, and celebrate successes. These gatherings strengthen team bonds and reinforce the collective purpose.
Team-Building Activities:
Organize team-building activities that encourage collaboration, communication, and problem-solving. Such activities enhance teamwork and help members understand each other better.
Team Traditions:
Establishing team traditions, whether it’s celebrating birthdays or achievements, fosters a sense of continuity and strengthens the team’s unique identity.
Building team identity is a crucial aspect of creating a successful and harmonious work environment. When team members identify strongly with their group, it enhances communication, morale, productivity, and resilience.
Lanyards, as a tangible representation of team membership, play a role in fostering this identity by creating a sense of belonging and unity among team members. However, it is essential to complement lanyards with other team-building efforts, such as defining a team charter, conducting regular meetings, and organizing team-building activities, to create a truly cohesive and motivated team.
* * *
By Alan, on July 29th, 2023 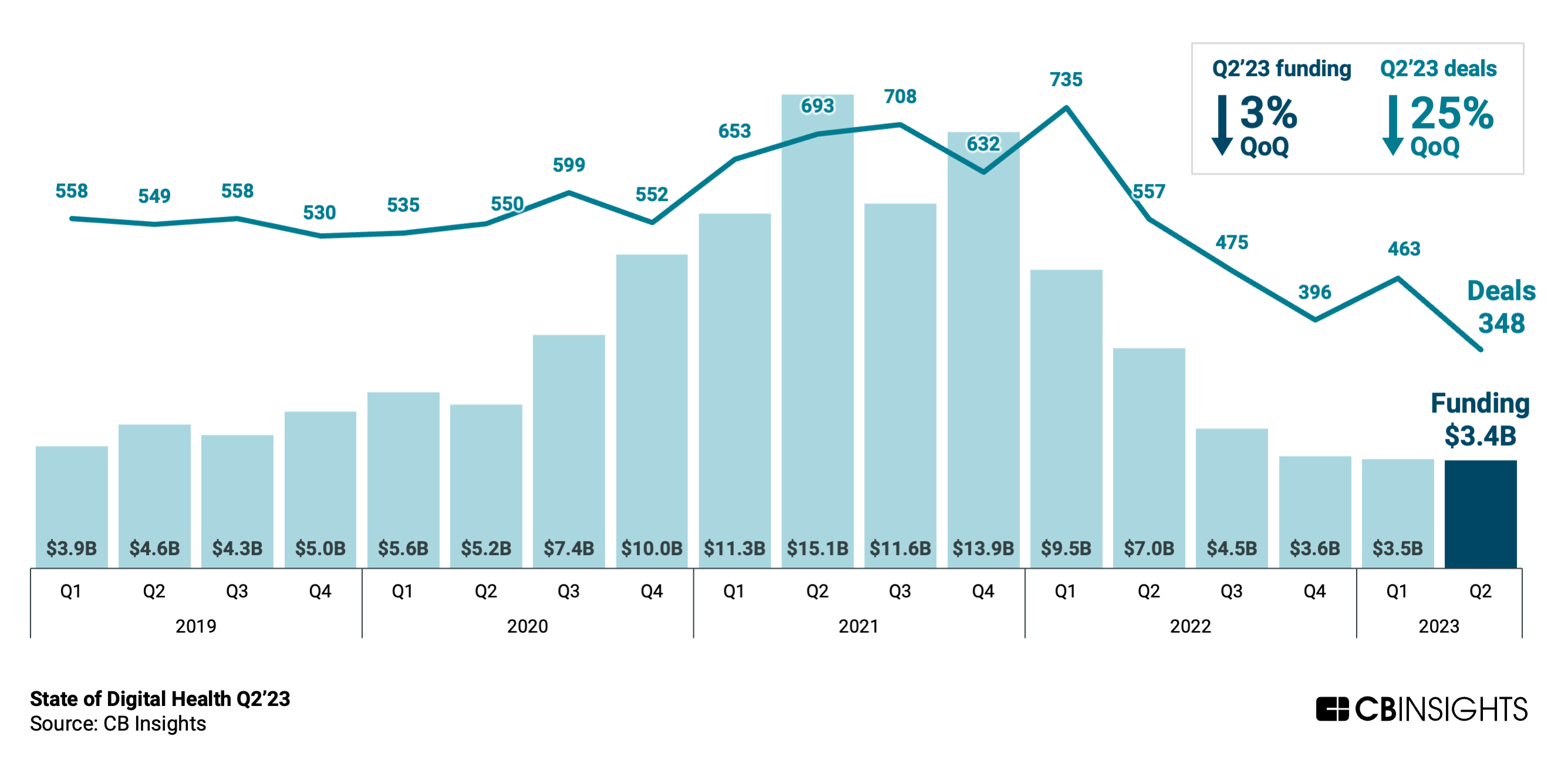 Click on image for full-size view (CB Insights) 29 July 2023. Global venture funding for digital health start-ups remains mired at low levels, as the number of investment deals in the second quarter of 2023 dropped to its lowest level in eight years. Technology intelligence company CB Insights reported the data earlier this week (subscription required).
CB Insights says digital health entrepreneurs worldwide raised $3.4 billion from venture investors in April through June, roughly the same total dollars raised as in the previous two quarters. Compared to venture investments across all industries that dropped 13 percent in the second quarter — or an 18 percent drop, as Science & Enterprise cited from Crunchbase’s report — a leveling-off of investment dollars is not bad. But the number of venture deals fell by 25 percent from Q1 to 348, the lowest quarterly deal total since Spring of 2015, according to CB Insights.
In the U.S., venture dollars for digital health start-ups in Q2 2023 totaled $2.2 billion in 174 deals, declines of eight percent and 25 percent respectively from the first quarter. Nonetheless, the U.S. companies account for about two-thirds (65%) of all investment dollars in this sector and half of the deals. Care delivery and navigation technology start-ups, such as primary care support and telehealth, raised $1.5 billion, 44 percent of the Q2 total, in 152 deals. Companies developing technologies for monitoring, imaging, and diagnostics collected $700 million in 77 Q2 deals.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|











 RSS - Posts
RSS - Posts
You must be logged in to post a comment.