Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on December 16th, 2019  Acme Market in Wayne, Pennsylvania (Albertsons Companies) 16 Dec. 2019. A chain of supermarket pharmacies in Pennsylvania will begin offering genetic tests to help customers manage their mental health medications. Acme Markets pharmacies in Pennsylvania, part of the Albertsons Companies chain, along with some Albertsons pharmacies in Idaho, will begin providing the Professional PGx Express test developed by Genomind in King of Prussia, Pennsylvania.
The Genomind Professional PGx Express test is given to help guide decisions by doctors and pharmacists on prescribing appropriate medications to treat mental disorders. Genomind cites data from National Alliance on Mental Illness showing one in five American adults (19%) experienced some form of mental illness in 2018. In addition, one in 25 adult Americans rate their mental illness as serious. A deadly outcome of mental illness is suicide, which now ranks as the second leading cause of death in people age 10 to 34.
The Professional PGx Express, which requires a prescription, uses samples from a patient’s cheek swab to analyze 24 genes associated with mental illness. A genetic analysis from the samples is then combined with a patient’s medical history to guide decisions by clinicians on appropriate treatments for the patient. The company says the data can help guide therapies for depression, anxiety, autism, schizophrenia, chronic pain, bipolar disorder, obsessive-compulsive disorder, attention deficit hyperactivity disorder, post-traumatic stress disorder, and substance abuse. A separate scaled-down test of 15 genes evaluates genetics related to anxiety and depression.
The company says it returns Professional PGx Express results in three days, for review by the physician and consultation with the patient and pharmacist. Results from the test include data on the patient’s unique genetics as well as traits associated with the genetic analysis, which influence the choice of medications. Patients can also gain access to the Neuroflow platform, an analytical technology for behavioral health that makes possible remote patient monitoring. Genomind and Neuroflow began its collaboration in August 2019.
The new agreement expands an initial partnership with Albertsons Companies begun in May 2018. In the first collaboration, Genomind provided its tests to 28 supermarket and retail pharmacies in or around Philadelphia and Chicago, as well as in Idaho. The new agreement more than doubles that number to 59 stores. In addition, participating pharmacies will gain access to Genomind’s G-DIG software that assesses proposed medications with other drugs prescribed for the patient, as well as effects of smoking or coffee drinking on drug metabolism.
“Pharmacists serve the front lines of medication management,” says Genomind CEO Shawn Patrick O’Brien in a company statement, “and this joint effort enables a coordinated team of clinicians, pharmacists, and pharmacogenomics experts to quickly and thoroughly address patient needs.”
Genomind cites data published in May 2018 from the Journal of Depression and Anxiety that its genetic tests to guide medication decisions for people with mood or anxiety disorders are associated with fewer subsequent hospitalizations and lower overall medical costs. Researchers assessed health insurance claims data from samples of people receiving genetic tests and matched the results to a comparable group without the tests. Those receiving the tests also had 40 percent fewer emergency room visits, 58 percent fewer in-patient hospitalizations, and saved an average of $1,948 in medical costs over six months.
More from Science & Enterprise:
* * *
By Alan, on December 14th, 2019 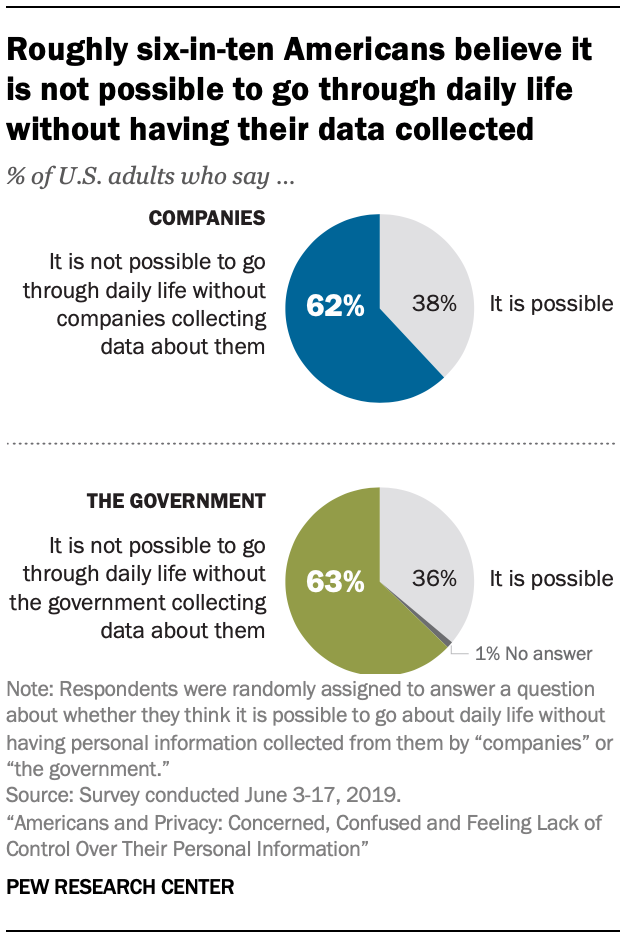 (Pew Research Center) 14 Dec. 2019. A solid majority of Americans says companies or government agencies are collecting data about them each day, and they’re not happy about it. The Pew Research Center reported the findings last month, based on a survey panel of 4,272 adults in the U.S. in June 2019.
More than six in 10 Americans tell the Pew researchers that it’s not possible to go a day without private companies (63%) and government (62%) collecting data about them, illustrated in this weekend’s infographic. Less than four in 10 in each case, 38 and 36 percent respectively, say it is possible to go through their daily lives without being tracked by companies or government agencies.
In addition, large majorities, from six to more than eight in 10 American adults, feel collecting data about their lives is getting out of control. Some eight in 10 in the U.S. feel they have little or no control over the data being collected about them by companies (81%) and government (84%). Moreover, from two-thirds to eight in 10 adults believe the risks outweigh the benefits, while about the same number are very or somewhat concerned about data collection, with private companies generating more negative reactions than government. And six to eight in 10 Americans do not understand what companies or the government do with the data they collect.
Many recent innovations in research technology reported in Science & Enterprise rely on smartphones or wearables to collect data routinely from participants. These findings suggest researchers need to be more open and transparent about their objectives and methods to gain participants’ cooperation.
More from Science & Enterprise:
* * *
By Alan, on December 13th, 2019  A new wrap material repels everything that comes into contact with it, including liquids and bacteria. (Georgia Kirkos, McMaster University) 13 Dec. 2019. A Canadian engineering team developed a treated form of common plastic that in lab tests repels liquids and stubborn bacterial films. Researchers from McMaster University in Hamilton, Ontario describe their discovery they call Repel Wrap in today’s issue of the journal ACS Nano (paid subscription required).
The team led by McMaster engineering physicist Leyla Soleymani and mechanical engineering professor Tohid Didar is seeking feasible solutions for health care facilities and homes to prevent the spread of bacteria becoming increasingly resistant to antibiotics. The problem of antimicrobial resistance, notes World Health Organization is global and growing. The organization says new mechanisms of resistance to antibiotics are emerging that threaten our ability to combat infections from pneumonia, tuberculosis, blood poisoning, gonorrhea, and foodborne diseases, particularly those contracted in health care facilities.
The Soleymani and Didar labs study materials for biomedical applications, and in this case are seeking solutions that can be implemented quickly and easily by health care facilities. The researchers based their solutions on common polymer plastics, like those found everyday household and health care uses, such as polyolefins and polystyrene. The team altered the chemical and physical structure of the polymers to create nanoscale ridges in the material’s surface.
The tiny ridges in the Repel Wrap’s surface makes the material hydrophobic so it repels water, blood, and other liquids. “We’re structurally tuning that plastic,” says Soleymani in a university statement released through EurekAlert. “This material gives us something that can be applied to all kinds of things.” Chemical modifications, say the authors, help keep the material flexible, durable, and inexpensive to produce.
The nanoscale ridges in the surface also enable the polymer to repel bacteria, including microbes that form into biofilms, or stubborn bacterial communities, by preventing the bacteria to anchor to the material’s surface. Biofilms are bacteria that connect and expand through a matrix of organic matter. These colonies also stick tightly to surfaces, including medical devices and implants as well as human skin and teeth, making them difficult to treat, because of their persistence and ability to resist conventional antibiotics.
The researchers tested Repel Wrap with two kinds of bacteria known for resistance to antibiotics and that form into biofilms: methicillin-resistant Staphylococcus aureus, or MRSA, and Pseudomonas aeruginosa. MRSA is a gram-poistive bacterium, while Pseudomonas aeruginosa is gram-negative. “Gram” refers to a classification for bacteria where the microbes either retain (gram-positive) or shed (gram-negative) a test stain on their protective cell coatings.
Tests in the lab show Repel Wrap sharply reduces biofilm formation with these bacteria, reducing MRSA biofilms by 87 percent and Pseudomonas aeruginosa colonies by 84 percent. Other tests show samples of the material touched by surfaces contaminated by E. coli bacteria, repel these microbes and remain bacteria-free.
“We can see this technology being used in all kinds of institutional and domestic settings,” notes Didar. “As the world confronts the crisis of anti-microbial resistance, we hope it will become an important part of the anti-bacterial tool box.”
The authors are seeking industry partners to develop commercial applications of the material. Soleymani and Didar tell more about and demonstrate Repel Wrap in this McMaster video.
More from Science & Enterprise:
* * *
By Alan, on December 13th, 2019  (commonfund.nih.gov) 13 Dec. 2019. Researchers in Sweden developed a process to improve the targeting of benign viruses for delivering gene therapies to the brain. Neuroscientists from Lund University describe their techniques in the 9 December issue of the Proceedings of the National Academy of Sciences.
A team from the molecular neuromodulation lab of Tomas Björklund at Lund University is seeking to improve the ability of viruses to better identify their targets among cells in the brain for delivering gene therapies. Many gene therapies, including gene-editing techniques such as Crispr, deliver healthy or modified genes with adeno-associated viruses. The adeno-associated virus is a benign and naturally occurring microbe that infects cells, but does not integrate with the cell’s genome or cause disease, other than at most mild reactions.
In their natural state, however, adeno-associated viruses or AAVs are imperfect delivery vehicles for gene therapies. One drawback is the ability to find the precise cells to deliver the modified genes, which reduces their efficacy and may result in off-target adverse effects. That’s a key concern for neuroscientists developing treatments for conditions such as Parkinson’s disease, where therapies need to identify and deliver gene therapies to dopamine-producing neurons, or nerve cells.
To improve this targeting ability, Björklund and colleagues devised a process for altering the protein shell on the virus known as the capsid to better reflect the molecular make-up of the cells needing the treatment. The researchers call their technique “Brave,” short for barcoded rational AAV vector evolution. The Brave process modifies the virus’s capsid to add a peptide, or short chain of amino acids known to interact with neurons to the capsid surface. The process, aided by a statistical model, also adds a unique molecular identifier — i.e., bar code — derived from RNA representing the genome sequence for repair.
With this process, the Lund team prepared a library of nearly 4 million combinations of peptides and bar codes that the authors say can map to specific binding sequences of proteins, and thus offers precise targeting for gene therapies with adeno-associated viruses. The researchers first validated their modified viruses to deliver gene therapies to dopamine-producing neurons derived from stem cells in lab cultures, then in lab rats transplanted with human dopamine-producing neurons, as well as nerve cells producing the toxic tau proteins and amygdala neurons associated with anxiety.
“Thanks to this technology” says Björklund in a university statement, “we can study millions of new virus variants in cell culture and animal models simultaneously. From this, we can subsequently create a computer simulation that constructs the most suitable virus shell for the chosen application, in this case, the dopamine-producing nerve cells for the treatment of Parkinson’s disease.”
Björklund is a co-founder of Dyno Therapeutics, a start-up company in Cambridge, Massachusetts, synthesizing viral capsids for precise delivery of gene therapies. As reported last month in Science & Enterprise, researchers from Harvard’s Wyss Institute and Dyno Therapeutics developed synthetic viruses to deliver gene therapies to the spleen, liver, kidneys, heart, and lungs in lab mice.
More from Science & Enterprise:
* * *
By Alan, on December 12th, 2019  Psilocybe aztecorum, also known as “magic mushrooms,” the source of psilocybin (Alan Rockefeller, Wikimedia Commons) 12 Dec. 2019. Results from an early clinical trial testing an hallucinogenic drug to treat depression shows the therapy is largely safe and feasible to administer. Findings from the trial testing the treatment code-named COMP360 by Compass Pathways in London were reported yesterday at a meeting of American College of Neuropsychopharmacology in Orlando, Florida.
Compass Pathways develops treatments for depression and other mental illnesses from psilocybin, an hallucinogenic compound found in so-called magic mushrooms, and considered a controlled substance under drug laws in the U.S. While psilocybin is not naturally addictive, it is used as a recreational drug to generate feelings of euphoria and sensory distortion. The drug can also trigger disturbing hallucinations, panic attacks, and anxiety, and is considered a risk for individuals with a family history of schizophrenia or early onset mental illness.
COMP360 is a derivative of psilocybin for people with depression that does not respond to current treatments. Compass Pathways was founded in 2016 by physician and medical researcher Ekaterina Malievskaia and technology entrepreneur George Goldsmith, a wife and husband team who experienced dealing with depression in a family member. Development of psilocybin into a treatment for depression is based on earlier research with the substance in the U.S. at Johns Hopkins University, New York University, and UCLA, as well as institutions in the U.K. and Switzerland. In October 2018, FDA designated COMP360 as a breakthrough therapy for treatment-resistant depression.
In the conference paper, Compass Pathways reported on an early-stage study of COMP360 at King’s College London with 89 participants evaluating its safety and feasibility factors with healthy adult volunteers. The study was led by researcher James Rucker in the Kings College department of psychological medicine that conducts trials of psilocybin.
In this trial, volunteers were randomly assigned to receive COMP360 in doses of 10 or 25 milligrams, or a placebo, followed by support in up to six simultaneous groups from trained clinicians for about six hours. The company says results show participants receiving COMP360 experienced no negative effects on cognitive or emotional functioning, nor any serious adverse effects. Individuals receiving COMP360 report some psychedelic adverse effects, such as changes in sensory perception and mood. The study team also says COMP360 can be feasibly administered in controlled settings, such as the group sessions used in the trial.
“This is the largest controlled study of psilocybin to date,” says Rucker in a company statement issued through PR Newswire. “The results of the study are clinically reassuring and support further development of psilocybin as a treatment for patients with mental health problems that haven’t improved with conventional therapy, such as treatment resistant depression.”
Compass Pathways also has a mid-stage clinical trial of COMP360 underway enrolling 216 participants in the U.S. and Europe with treatment-resistant depression. The trial is looking primarily at indicators of the drug’s safety, as well as scores by patients on a standard rating scale of depression symptoms from clinicians.
More from Science & Enterprise:
* * *
By Alan, on December 12th, 2019 – Contributed content –
 (LinkedIn, Pexels) 12 Dec. 2019. Keeping your patients safe, and anything they tell you a secret, is something a medical business needs to hold in high regard. When someone walks through your door, looking to book an appointment and be handled with care, they need to be able to rest assured that whatever they say in the office, and any results for diagnosis given to them, will be kept safe and confidential.
And if you’re looking to start a medical business of your own, it’s one of the most important things to keep in mind during your planning stages. How are you going to keep your patient files confidential, and still ensure you’re running with all the access and affordability you need to?
With the new improvements in technology, and the amount of security threat that could potentially have, is your medical business at risk? After all, hackers are becoming more and more sophisticated in their attempts, and it’s something that’s very much in patient mind these days.
Confidentiality: what defines it
Your patients need to be able to trust you. And if you want to do the best for your patients, you need to be able to prove this to them. Otherwise the people who come to your door, seeking aid, may not report all of the symptoms they’re experiencing, or not even seek the help they need in the first place. And that’s a very bad turn of events for the healthcare sector and anyone looking to move into it.
Of course, in the modern day and age, the doctor’s job is a lot different to what it used to be. Nowadays doctors can be mediators and advisors, alongside medical professionals, and their work is brought up in a wide circle of other sectors, such as social care. These elements need to be factored into your confidentiality policy, when putting one together, to ensure you’re upfront about the relationship you’ll be able to hold with any one of your patients.
At the same time, other professionals will sometimes need access to patient records. Nurses, and other auxiliary services, or even just home care services for older or disabled people, need to be able to read through patient records when taking on a new case. So, access needs to be allowed to the right people, with the right credentials, so the record keeping system needs to be heavily monitored, and properly administered. Keep this in mind when you’re looking to hire someone to manage both the digital and physical system.
Overall, as a doctor, nurse, consultant or other medical professional, you have ethical and legal responsibilities. This will vary from area to area, but finding a fine line between the two elements of providing for your patients and acting within the law takes some working. Make sure you have the time in your schedule to trial your way to the right system.
The issue of keeping family records
Pediatricians alone have a lot on their plate, being a family friendly doctor dealing with serious issues on a regular basis. If you’re running a pediatric enabled business, and you seek to serve families in your area, you might come across a few issues being able to keep records as confidential as you would like them to be. After all, depending on the age of the child whose record you’re keeping, they may not want their parents to be informed of potential issues they may be suffering with.
At the same time, if a parent is suffering with something that may be genetic, respecting patient confidentiality and their wishes may require you to keep the potential for inheritance from their children. This can become an issue as they age.
How do you improve upon a situation like this? They’re far more common than you may think; indeed, very recently, a report of a woman not being told about her father’s Huntington Disease has been in the media. It’s a rough situation for everyone involved, and can bring a harsh amount of criticism on the parties involved, including the doctors simply keeping to the rules of their jobs.
Are some breaches justified?
As we mentioned above, some patient confidentiality breaches dance the line between ethical and unethical, and it’s hard to find the right answer between the two. Every patient who comes to you has the automatic right to keep their records to themselves, but if the information within is sensitive for more than one person in question, it may be justified to allow access.
Some cases seem cut and dry, and little to no patient cooperation is required to hand over details. Some cases may require a doctor to allow the police access to a patient’s file, if they’re suspected of being an associate or taking part in a recent crime in the area. If it’s reported to the authorities that someone has been seen being treated for wounds concurrent with what they may have sustained at the scene, access is often applied for.
But in plenty of other cases, patient consent must be obtained first, before anyone else is allowed access to a certain medical record of theirs. Patients must be walked through the reasons why a third party is asking for access, and why this may be significant to anyone involved. In a case like this, the patient comes first, and their wishes are respected.
Starting a new medical business with patients in mind
If you have the plans for a medical business of your own, you’ll have to take your time with the ethics and responsibilities involved. It can seem like quite the grey area, but you’re not going to be the first to have to face it; there are always going to be guidelines in place for whether or not the patient confidentiality model is allowed to be broken.
Of course, you can rely on your own knowledge and experience here too, and as a doctor, you’ll be a valued part of the procedure. Keep this in mind.
Editor’s note: The opinions in this post are the contributor’s and not those of Science & Enterprise.
* * *
By Alan, on December 11th, 2019  Coil made with magnetic shape polymer material (Allison Carter, Georgia Tech) 11 Dec. 2019. Researchers created a new soft polymer plastic with magnetic properties that can be programmed to change shape and recall previous shapes. The material — with applications in robotics, electronics, and medical devices — is described in the 8 December issue of the journal Advanced Materials (paid subscription required).
A team from Georgia Institute of Technology in Atlanta and Ohio State University in Columbus developed the magnetic shape memory polymer to provide a combination of desirable properties in a single composite material. Engineers and materials scientists from the labs of Jerry Qi at Georgia Tech and Ohio State’s Ruike Zhao are seeking a flexible and pliable substance that can be programmed to change shape quickly, yet revert to its previous shape if needed.
The Georgia Tech-Ohio State team started with shape memory polymers, plastic materials that change their shape in response to heat, light, or other stimuli, yet can be restored to their previous shape. Shape memory polymers are used in a range of applications including smart fabrics, electronics, mobile phones, and implanted medical devices. Science & Enterprise reported in September 2018 on shape memory materials housing electronics to provide robotics functions to inanimate objects.
In their paper, Qi, Zhao, and colleagues add magnetic particles to shape memory polymers to provide a magnetic response for producing heat to change the material’s shape, as well as programming ability, which are restricted in many current robotic materials. “The degree of freedom is limited in conventional robotics,” says Zhao in a Georgia Tech statement. “With soft materials, that degree of freedom is unlimited.”
To provide these features, neodymium iron boron, a commercially-available magnetic material, and iron oxide particles are distributed through the polymer. When exposed to a magnetic field, the magnetic particles begin to heat, and from that heat, the polymer becomes soft and pliable, allowing it to change shape. After stopping the magnetic field, the polymer cools and returns to its hardened form.
In bench tests, the researchers shaped the polymer into a coil, with the material expanding and contracting. The team stretched and retracted the coil to simulate an antenna changing frequencies, in response to various magnetic fields. The researchers also added a second magnetic field to operate a robotic gripper. The first magnetic field softened the polymer material in a T-shaped mold, to make it pliable. The second magnetic field then controlled the opening and closing of the gripper.
“We envision,” says Qi, “this material being useful for situations where a robotic arm would need to lift a very delicate object without damaging it, such as in the food industry or for chemical or biomedical applications.” The researchers note that the material changes shapes in just a few seconds, and when the material cools, the gripper can lift items up to 1,000 times its own weight.
“This is the first material,” adds Qi, “that combines the strengths of all of these individual components into a single system capable of rapid and reprogrammable shape changes that are lockable and reversible.” This Georgia Tech video demonstrates a few applications of the material.
More from Science & Enterprise:
* * *
By Alan, on December 11th, 2019 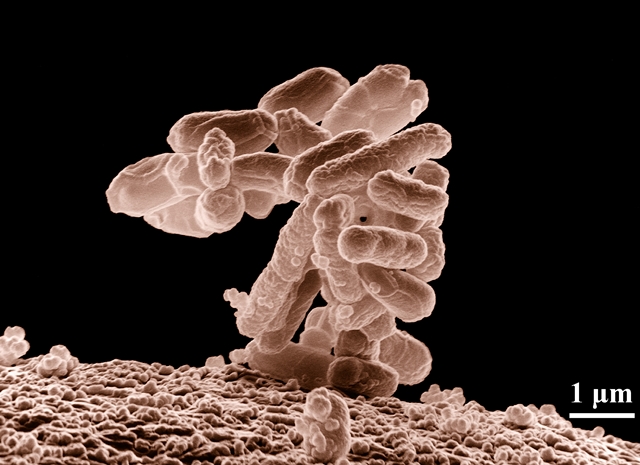 E. coli bacteria (Agricultural Research Service, USDA) 11 Dec. 2019. A company developing protein-like therapies for cancer from synthetic bacteria is being acquired by drug maker Sanofi in a deal valued at $2.35 billion. Synthorx Inc. in La Jolla, California is a five year-old spin-off enterprise from the Scripps Research biochemistry lab of Floyd Romesberg, now with Synthorx full time.
Synthorx develops biologic drugs from genetically altered but benign E. coli bacteria, a well-researched microbe that in some strains is known to cause food poisoning. The Romesberg lab at Scripps Research developed techniques for adding two more base-pairs, nucleic acid components configured into an organism’s genetic code, to DNA. Natural DNA has four base pairs, combinations of adenine (A), thymine (T), cytosine (C), and guanine (G). Amino acids are made from sequences of three of these components, which form the building blocks of proteins in organisms with instructions for cells.
Romesberg and colleagues found a way of adding the two base pairs, labeled X and Y, to DNA. With these extra base pairs, new kinds of proteins can offer additional properties for therapeutics beyond the capability of proteins in their natural form. The added base pairs make it possible to encode up to 152 new amino acids for proteins with unique chemical characteristics for therapies or other applications.
Synthorx adapts this technology to create E. coli bacteria with altered DNA for producing what the company calls synthorins, biologic drugs with protein-like therapeutic characteristics, but also properties from the X and Y base pairs. These added properties include features such as longer active life in the body, and greater uptake and retention by targeted tumors.
The company’s lead product, code-named THOR-707, is a synthorin biologic for treating solid tumor cancers. THOR-707 is a synthetic form of the natural protein Interleukin-2 in the immune system that produces an immune response to attack tumor cells. In THOR-707, however, Synthorx modifies the protein’s chemistry to act for longer periods, spread wider in the tumor, and be retained longer in the tumor than natural Interleukin-2. In addition, says the company, THOR-707 does not cause vascular leak syndrome, a weakening of blood vessels that in the past limited use of Interleukin-2 as a cancer therapy.
THOR-707 is being tested in an early- and mid-stage clinical trial as a treatment for solid tumor cancers, both on its own and with immune-system checkpoint inhibitors. The trial is enrolling 300 participants in the U.S. and Australia with advanced or metastatic solid tumor cancers, looking primarily for safe doses of THOR-707, but also clinical responses to the treatments and survival times by patients.
Sanofi expects THOR-707 and other therapies in development by Synthorx to bolster its current pipeline of cancer immunotherapies and synthetic biologics. Sanofi is purchasing Synthorx stock for $68.00 a share, a premium of 172 percent over Synthorx’s closing price on 6 December. The all-cash transaction is valued at $2.35 billion.
Science & Enterprise reported on Synthorx’s founding by Romesberg and others in May 2014, and publication in November 2017 of key advances in producing proteins from synthetic bacteria in the journal Nature. In August of this year, Romesberg abruptly left Scripps Institute under largely unexplained circumstances, according the Science magazine. His lab continues at Scripps, although the institute says it’s winding down the lab’s work.
More from Science & Enterprise:
* * *
By Alan, on December 10th, 2019  Matteo Pasquali holds a spool of fibers made from carbon nanotubes (Jeff Fitlow, Rice University) 10 Dec. 2019. Rice University and the energy company Shell launched a program to sustainably produce carbon derived from splitting rather than burning hydrocarbons. The Carbon Hub, as the project is known, is financed initially by a $10 million grant from Shell, but the program aims to expand to 20 institutions, supporting 70 researchers, and conducting basic science and engineering valued at $100 million.
The Carbon Hub is seeking new carbon-based products adding no greenhouse gas emissions that accelerate the impending climate crisis. Current efforts to derive clean hydrogen fuel from splitting hydrocarbons, say the partners, result in three tons of solid carbon for each ton of hydrogen produced. The Carbon Hub, on the other hand, aims to find ways of producing carbon-based products directly from hydrocarbons with zero emissions, leaving hydrogen as a by-product.
“Finding a productive use for carbon at a very large scale is the key,” says Matteo Pasquali, a chemical engineering professor at Rice in Houston and director of the Carbon Hub, in a university statement, “and the novelty of the Carbon Hub is that we’re going to do something very useful with the carbon.” Pasquali’s lab studies the behavior of molecules at nano- to microscale dimensions, including carbon nanotubes and fibers.
The Carbon Hub aims to build on these advances, with practical applications for carbon-based products in construction and transportation. The payoff for the climate, say the researchers, is development of materials in construction and transportation that are lighter than current metals, but at least as strong.
Applications extend as well to agriculture. “One potential use of solid carbon is as a soil additive,” notes Carrie Masiello, professor of Earth, environmental and planetary sciences, “where properly engineered carbonaceous materials can improve crop yields and resilience to drought.” Masiello is also one of the Carbon Hub researchers.
In addition to new product development, the Carbon Hub expects to investigate supply chain implications of sustainable carbon, as well as the need for new policies and community engagement to support advances in technology. Pasquali says that without attention to these factors, the effects of new technologies on climate change can be wasted.
“For example,” Pasquali adds, “you make cars more fuel efficient by removing weight, and then realize you’ve increased CO2 emissions by using more aluminum and carbon fibers. Or you try to fix CO2 into a useful product, and you realize you now need much more energy than you had gotten by making the CO2 in the first place”
Sharon Beshouri, president of Shell Global Solutions, notes that the Carbon Hub can play a key role in the company’s transformation from mainly an energy supplier. “The use of clean hydrogen and carbon materials,” says Beshouri, “has the potential to be game-changing in the energy transition. Carbon Hub aligns with Shell’s vision to provide more and cleaner energy solutions around the world.”
More from Science & Enterprise:
* * *
By Alan, on December 10th, 2019 – Contributed content –
 (MaxPixel.net) 10 Dec. 2019. One of the main goals of business is to grow it, and as long as this is happening – at whatever rate it might be happening – you can be sure that things are moving in the right direction. But the truth is that a lot of business owners are not really clued up on what expanding a business looks like, and without that knowledge to hand it is just going to be much more difficult to know when it’s happening or what to do about it when it does start happening. In this article, we are going to look at some of the major ways that you might expand a business, and what you can expect to happen when you do.
Opening another location
Probably one of the simplest ways to expand a business is to open up a second location, or a third, or whatever it might be for you at this time. This is both a very simple method and also one fraught with danger and difficulty, as it is a lot of pressure being put on to one very small part of a business. If anything goes wrong, it could mean that the whole business struggles, and that is obviously not what you are going to want to happen here. However, it can be hugely successful, and if you want to expand your business, then opening up another location is going to be one of the best ways to go about doing just that. Just make sure that your business really is ready for it first.
Buy another business
If you are doing particularly well, then you might be in a position to buy another business, and make that a part of your own. This is something that happens mostly with the very largest corporations, but it is also something that can occur on a smaller scale, and with fewer funds, with smaller businesses. If you do want to think about buying another business, then you might want to make sure that you are choosing well, as it is going to affect your own business in a number of ways.
First of all, you are going to have to make sure that you can afford it. Some businesses buy others with cash, and sometimes it is done using stock instead. You can find more information on that, but generally you need to make sure that you are going to be in a good position to be able to purchase that other business. You also need to make sure that they have a good reputation, as you will become immediately tarred with the same brush if there is anything dark in their history.
As long as you have considered all that, this can be a great way to really give your business a lot more size, and to help it expand considerably quicker than in many other ways.
Overseas expansion
A particularly exciting way of expanding a business is to look into trying to expand it overseas. That might be because you feel you have mastered the market in your country and you want to do the same elsewhere, or because you have a sense of wanting to try your hand somewhere because things are not as good at home. In either case, however, you are going to need a strong base, so make sure that you are thinking about that from the start. If something goes wrong abroad, you need to make sure that you can focus on reeling it all back in to your home base in the original country, so if you can’t do that easily it might not be time to think about overseas expansion at all.
Offering more products
Sometimes, on the other end of the scale, expanding can be as simple as offering more products to your customers. Branching out in this way is often enough to ensure that your business is going to grow quickly, and especially if you have put a lot of effort into ensuring that you put out the right products in the first place. That means researching what is likely to be popular among your customers, and making sure that you offer them exactly a solution to that problem. If you can do that well enough, and do a good job on the marketing, it could mean great things for your business in no time.
As you can see, there are many ways to expand your business, so make sure that you are focusing on these when you feel the time is right.
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|









 RSS - Posts
RSS - Posts
You must be logged in to post a comment.