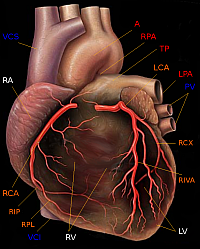
Medical device developer Medtronic Inc. in Minneapolis, Minnesota, said today the U.S. Food and Drug Administration (FDA) approved its Integrity coronary stent system for use in the United States. The stent system already received the CE (Conformité Européene) mark in February and is currently available in approximately 100 countries outside the United States.
The company says the new bare-metal stent is based on an advance in biomedical engineering called continuous sinusoid technology. Instead of rings, continuous sinusoid technology enables each stent to be made from a single wire, comparable to a flexible spring. The Integrity stent is made from cobalt alloy shaped into crowns and, to maximize flexibility, laser fused where only certain points on the crowns meet.
Medtronic says the Integrity system has been shown in bench testing and in blinded in vivo physician assessment studies to be highly deliverable. In this context, deliverability means the ability of the device to navigate the patient’s circulatory system and reach the narrowed heart artery targeted for treatment.

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.