Medical researchers at University of Edinburgh in Scotland created a process for inducing pluripotent stem cells to transform into liver cells with the same consistency and quality needed to test drugs for toxicity. A spin-off company from the university has also formed to take the research to market. The team led by Edinburgh’s David Hay published its findings online today in the journal Stem Cells Translational Medicine (paid subscription required).
Cells from human livers, known as hepatocytes, are used to test for drug toxicity, because of the liver’s key role in metabolizing compounds, with cultured heptocytes from liver tissue increasingly used for testing in the pharmaceutical industry. Cells derived from human liver tissue, however, are in short supply and often of varying quality because of different donors, which makes them an unreliable source for high-volume testing in commercial labs.
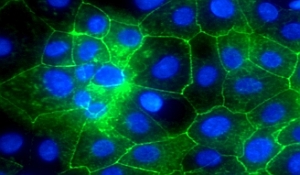
Hay’s team, which included colleagues from the pharmaceutical company Bristol-Myers Squibb in New Jersey, induced pluripotent stem cells — adult stem cells that can be genetically reprogrammed into an embryonic state — to transform into hepatocytes. The team discovered its process generated liver cells that remained stable in the lab for more than two weeks.
The researchers evaluated these stem cell-induced hepatocytes as test media for specific toxic compounds, and found the cells equal in sensitivity to these compounds as assays made with human liver cells found in today’s labs. The uniformity and consistency of the cells, would thus make them a reliable and predictable test culture for drug toxicity. Hay believes this method can also generate stem cells with different DNA, to reflect genetic variations in the way human livers metabolize compounds, and provide a way of predicting varying responses to certain drugs.
Hay is one of four founders of FibromEd Ltd., a biotechnology company spun-off from University of Edinburgh in 2011, developing hepatocytes and other human liver models. It’s first product is Hepatoinform, a stem-cell derived system for generating hepatocytes for human metabolite and toxicity screening.
Read more:
- Stem Cell Process Makes Red Blood, Platelets in Quantities
- University, Biotech Develop Heart Tissue Repair Patch
- Retinal Cells Produced Without Animal Matter from Stem Cells
- Nano Patterns in Plastic Help Stem Cells Become Bone Cells
- Stem Cells Induced to Become Blood Vessel Tissue Cells
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.