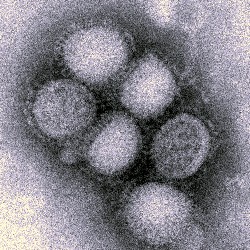
Bharat Biotech in Hyderabad, India said today it developed India’s first cell-culture H1N1 swine flu vaccine. The company believes it is the only developing world flu vaccine to be manufactured in cell culture, a faster and more advanced manufacturing process, instead of eggs.
Traditional egg-based flu vaccine production requires long lead times — four to six months in some cases — that makes it difficult for vaccine producers to respond to quickly emerging strains, such as last year’s H1N1 flu. With cell-culture techniques, there are no lead times involved, since typical cell-culture processes use cell lines rather than eggs. Once a cell line is infected with the seed virus in a fermenter, the process can begin.
The company says the vaccine, marketed under the name HNVAC, was tested in one of the Phase 1, 2, and 3 clinical trials for flu vaccines in India for safety and tolerance. The vaccine was developed with approved strains from World Health Organization and and the U.S. Centers for Disease Control and Prevention.

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.