The medical device manufacturer Medtronic Inc. in Minneapolis says the U.S. Food and Drug Administration on Friday approved a wearable artificial pancreas system that combines an insulin pump and sensor to detect glucose levels for people with diabetes. The company also revealed it received a warning letter on 19 September from FDA about its factory that makes diabetes-control devices, and the agency conditioned its approval on Medtronic addressing those issues.
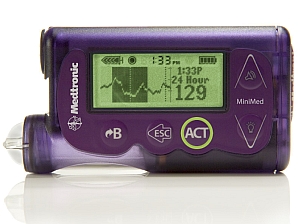
The MiniMed 530G is a battery-powered insulin pump with an infusion set attached to the body and a reservoir to hold the insulin supply. The system includes a sensor that detects when glucose levels reach a specified lower threshold and either sounds an alarm or, if the wearer is sleeping or unconscious, automatically stops delivery of insulin.The sensor can be worn for up to six days.
The sensor also gives warnings up to 30 minutes in advance of reaching high or low glucose levels. In addition, the system gives routine readings every five minutes for continuous glucose management by the patient. The software includes features to quickly calculate the need for immediate adjustments in insulin after eating, called bolus doses,.
Medtronic calls the device a first-generation artificial pancreas system, in that it requires occasional interaction with the patient to deliver the needed insulin. The company says it is the first system approved by FDA in a new classification of medical devices, Artificial Pancreas Device System — Threshold Suspend, product code OZO.
FDA approved the MiniMed 530G for people with diabetes age 16 and older. The agency’s approval is conditioned on Medtronic conducting a post-approval study for younger children to age 2, as well as direct follow-up with patients. Approval is also conditioned on Medtronic addressing concerns raised by FDA about the company’s manufacturing plant in Northridge, California that makes devices for people with diabetes.
The online industry newsletter Mass Device reported on Friday those issues were contained in a letter from FDA to Medtronic dated 19 September covering “corrective and preventative action, complaint handling processes, process validation, process monitoring, design control and general good manufacturing processes,” according to an e-mail from a company spokesperson. Medtronic says in a statement it already addressed many of the issues raised in the letter and is committed to resolving remaining issues specified by FDA.
Read more:
- Arthritis Drug Shown to Reduce Glucose in Type 2 Diabetes
- FDA Approves Type 2 Diabetes Drug, Orders More Studies
- Grant to Fund Glucose-Sensitive Insulin Development
- Clinical Trial to Evaluate Type 2 Diabetes Treatments
- SBIR Grant to Support Glucagon Made for Artificial Pancreas
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.