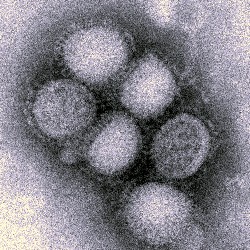
NanoBio Corporation in Ann Arbor, Michigan received a new contract from National Institute of Allergy and Infectious Diseases, part of National Institutes of Health, for development of a pandemic influenza vaccine and adjuvant to boost the immune response of that vaccine. The initial contract value is $5.5 million, but the company says it would be eligible for another $5 million if all options under the contract are exercised.
NanBio is a spin-off company from the University of Michigan Nanotechnology Institute for Medicine and Biological Sciences, founded in 2000. The company develops anti-infection compounds and vaccines using its technology that creates oil-in-water emulsions of nanoscale (150 to 400 nanometer) droplets, stabilized with surfactants. The nanoscale emulsions, says the company, can quickly penetrate skin pores and hair shafts to fight infections, or in this case, boost a flu vaccine’s potency.
Funds from this contract will be used for research and development on NanBio’s NanoVax-Panflu product that combines an engineered antigen producing antibodies targeting pandemic influenza with a nano-emulsion adjuvant to boost the immune response of the antigen. The company says this vaccine, administered as a nasal spray, has the potential for protecting against infection both on mucus membranes (e.g., in the nose) and the overall immune system.
The contract, says the company, includes an option to extend the R&D effort to a nanoscale emulsion adjuvant for a vaccine for HIV infections. The option would cover a test of engineered virus-like particles resembling HIV combined with nano-emulsion adjuvant.
In a statement, NanBio’s CEO David Peralta says the company recently completed studies of vaccines enhanced with nano-emulsion (NE) adjuvants for respiratory syncytial virus that affects the lungs and respiratory tract, as well as type 2 genital herpes, viruses that both enter the body through mucosal sites. “The results of these studies,” says Peralta, “very clearly demonstrate the ability of the NE adjuvant to elicit mucosal immunity and the important role this type of immunity plays in protecting against disease.”
Read more:
- FDA Approves Shipping of GSK Four-Strain Flu Vaccine
- Biotech Gets Small Business Funds for Universal Flu Vaccine
- FDA Clears Sanofi Four-Strain Flu Vaccine for Adults, Kids
- Algorithm Identifies HIV Antibodies For Vaccine Design
- FDA Approves Flu Vaccine Based on Engineered Viruses
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.