Bacterin International Holdings, a medical device company in Belgrade, Montana received a patent for its technology to produce a form of human bone material suitable for bone grafts that promote regeneration of bone tissue. The U.S. Patent and Trademark Office yesterday issued patent 8,574,825 to seven inventors, including Gregory Juda, Bacterin’s chief scientist, and Steven Scott, who chairs the company’s scientific advisory board, and assigned the patent to the Bacterin.
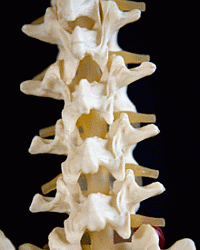
The patent covers the process for demineralizing bone matter used in bone grafts for procedures such as spinal fusions that encourage the growth of bone tissue to fuse vertebrae in the spine, thus limiting the movement and pain from degenerating discs between vertebrae. The patent document notes that some 1 million bone graft procedures are conducted each year, with about half of those procedures in the U.S.
Demineralized bone matrix is useful when the patient cannot provide his or her own bone matter for a bone graft. However, bone matter from a donor runs risks of rejection or potential infection, as well as less desirable structural properties, such as porosity or density. In addition, synthetic graft materials use putty or gel fillers, which also may not have the same characteristics of natural bone, particularly when those characteristics can vary among different parts of the body.
The process described in the patent subjects donor bone material to an acid solution that removes minerals, while protecting the growth factors that stimulate tissue growth. The patented process allows for periodic pauses to allow for tests of compression or other mechanical properties, and to switch to less acidic solutions until the desired properties are reached.
Bacterin expects to apply the patent to production of its OsteoSponge product, a bone graft material made from human bone, without putty or gel fillers, but is also compressible to allow for better fit during surgical implantation. The company says OsteoSponge provides more surface area as well as signaling molecules that trigger formation of new bone.
Read more:
- Nanowire Coating Boosts Bone Bonding to Implant Material
- 3-D Printing, Computer Model Generate Synthetic Bone Matter
- Biotech, University to Partner on Bone Regeneration Trial
- Nano Patterns in Plastic Help Stem Cells Become Bone Cells
- Stem Cell Method Developed to Increase Bone Strength
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.