22 September 2014. A Cambridge, Massachusetts diagnostics company received a Small Business Innovation Research (SBIR) grant to develop a simple point-of-care test for sickle cell disease, a genetic blood disorder affecting a large percentage of people of African origin. The $225,000 grant from National Institutes of Health’s SBIR program to Daktari Diagnostics will support development and testing of a commercial prototype of the sickle cell diagnostic, in partnership with Harvard University, University of South Carolina, and University Teaching Hospital in Lusaka, Zambia.
Sickle cell disease is a genetic blood disorder affecting hemoglobin that delivers oxygen to cells in the body. People with sickle cell disease have hemoglobin molecules that cause blood cells to form in an atypical crescent or sickle shape. That abnormal shape causes the blood cells to break down, lose flexibility, and accumulate in tiny capillaries, leading to anemia and periodic painful episodes. The disease is prevalent worldwide, and affects 70,000 to 80,000 people in the U.S., including about 1 in 500 people of African descent.
Newborns in the U.S. are tested routinely for sickle cell disease, but not in many areas where resources are limited, including many places in Africa. Daktari Diagnostics develops medical tests for resource-limited regions, combining microfluidics (lab-on-a-chip) technologies with electrochemical sensing, which the company says are designed to be simple, portable, and inexpensive.
The grant — from NIH’s National Heart, Lung, and Blood Institute — supports development of a commercial prototype based on a device designed in the lab of Harvard chemistry professor George Whitesides by then-graduate student A. J. Kumar. The system devised by Kumar and colleagues harnesses a principle in chemistry that polymer chemicals separate into different layers when mixed with water. The team applied that idea to separating red blood cells of different densities in a mix of polymers and water.
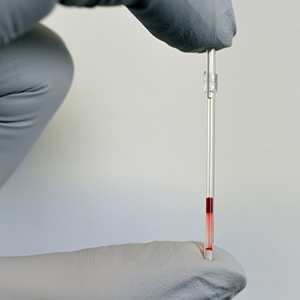
Kumar, now a postdoctoral researcher in the Whitesides lab and the lead engineer on Daktari’s project, tested an early version of the device as a proof-of-concept exercise, with the results published earlier this month in the journal Proceedings of the National Academy of Sciences. The device basically consists of a narrow tube with a mix of water and polymers, where a drop of blood is drawn with a finger prick that wicks into the tube. Spinning the tube on a standard lab centrifuge separates the multiphase blood-polymer-water mixture into visually discernible layers, with the heavier sickle cells sinking to the bottom, all in about 12 minutes.
For the commercial prototype Daktari aims to develop a device with tubes designed for a simple, battery-powered centrifuge, pre-filled with the polymer-water mixture. Blood samples would be taken with a finger-prick or a heel-stick from newborns. If sickle cells are present in the blood, they will sink to a designated region in the tube, where technicians can easily identify them. As with the proof-of-concept, Daktari is aiming to have the commercial test return results in about 12 minutes.
A key part of the proposed commercial system is the simple battery-powered centrifuge to separate the blood-water-polymer mixture into layers. Daktari says it plans to apply its experience with a battery-powered, hand-held device to measure CD4 T-cells in blood as part of an HIV test.
Daktari says the test is called Mpana, an acronym for MultiPhase Analyzer, but also a Swahili word for ” a broad, wide, open channel.” The grant funding covers work on the device through May 2015, although the project is expected to continue through May 2016.
Read more:
- Small Business Contract to Fund Cancer Drug Response Tests
- Synthetic Platelet-Like Particles Developed, Tested
- Smartphone App Screens Infants for Jaundice
- FDA Approves AFib Monitor Algorithm for Mobile Devices
- Graphene Sensor Designed for Wearable Disease Detection
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.