23 December 2014. The U.S. Food and Drug Administration approved yesterday a drug to treat influenza infections in adults. The treatment, Rapivab, is a small molecule drug made by BioCryst Pharmaceuticals in Durham, North Carolina.
BioCryst designs and develops therapies that block enzymes involved in infectious and inflammatory diseases, with a technology that creates synthetic compounds using x-ray crystallography and computer modeling of molecular structures. The company says its use of crystallography allows for analyzing the three-dimensional structure of molecules, resulting in more precise targeting by its drug compounds.
Centers for Disease Control and Prevention reports the 2014-15 flu season is already underway with the proprtion of outpatients visits for flu-like symptoms at 3.7 percent, well above the national baseline of 2 percent. CDC expects between 5 to 20 percent of the U.S. population to get the flu, with more than 200,000 hospitalizations.
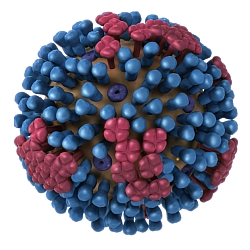
Rapivab is designed by BioCryst to block the activity of neuraminidase, an enzyme that releases virus particles from the surface of infected cells. The drug is given as an intravenous infusion in doses of 600 milligrams. FDA approved the drug for adults age 18 and over, who have influenza without complications and experience symptoms for no more than 2 days.
BioCryst developed Rapivab under contract to the Biomedical Advanced Research and Development Authority, a division of the U.S. Department of Health and Human Services. The company says Rapivab was tested in 27 clinical trials, and more than 1 million patients received the drug in Japan and Korea, where it was earlier approved. FDA says there are two other neuraminidase inhibitors, but Rapivab is the first of its kind approved for intravenous infusion.
FDA’s approval was based on a clinical trial with nearly 300 flu patients receving Rapivab in doses of 600 or 300 milligrams, or a placebo. The results show patients receiving 600 milligrams of Rapivab had their flu symptoms relieved 21 hours earlier and reducing fever to normal temperature 12 hours earlier than the placebo. The drug’s effectiveness could not be established, however, in patients with serious cases of flu requiring hospitalization.
The most common adverse effects is diarrhea, although in the clinical trial it occurred at about the same rate (8%) as the placebo (7%). Rare skin infections also occurred.
Read more:
- Ebola-Marburg Vaccine Development, Testing Contract Awarded
- FDA Exemption Sought for Ebola Blood Plasma Device
- Hepatitis C Combination Drug Approved by FDA
- U.S., Canada Authorities OK Biotech Ebola Treatments
- Trial Shows Single-Dose Antibiotic Effective with MRSA
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.