19 March 2015. The pharmaceutical company Eli Lilly and Company is acquiring from Hanmi Pharmaceutical, an experimental drug that blocks the actions of an enzyme associated with rheumatoid arthritis and other autoimmune diseases. The deal can pay Hanmi, in Seoul, Korea, as much as $690 million.
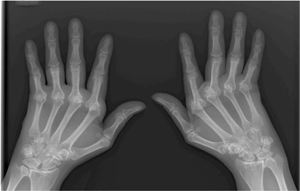
Autoimmune disorders are diseases where the body’s immune system is tricked into attacking healthy cells rather than invading bacteria or viruses. Among the better known autoimmune diseases are rheumatoid arthritis, multiple sclerosis, and lupus. Women have a higher risk for autoimmune diseases, which are often characterized by inflammation, resulting in swelling and pain.
Hanmi is developing a small molecule — low molecular weight — drug code-named HM71224 to treat autoimmune disorders. HM71224 limits the actions of an enzyme known as Bruton’s tyrosine kinase, or BTK, essential for the development and maturation of B cells, a type of white blood cell that helps protect the body against infection.
BTK proteins send chemical signals instructing B cells to mature and produce antibodies. In some cases, however, BTK enzymes can trigger autoimmune reactions from B cells, where blocking their activity may relieve the resulting inflammation, swelling, and pain.
Hanmi designed HM71224 as an inhibitor of BTK enzymes. The company tested the drug in an early-stage safety clinical trial with 58 healthy volunteers in the Netherlands. In June 2014, the company reported results from the trial indicating HM71224 was successfully absorbed into the body with single and multiple doses, and was not affected by food intake.
Under the agreement, Hanmi is providing a license to Eli Lilly and Company to develop and commercialize HM71224 worldwide, except for China, Hong Kong, Taiwan, and Korea. In return, Hanmi is receiving an initial payment $50 million, and is eligible for another $640 million in development, regulatory, and sales milestones. If commercial products are developed from the partnership, Hanmi can also receive royalties on their sales.
The companies say HM71224 is ready for intermediate-stage clinical trials as a treatment for rheumatoid arthritis, lupus, and other autoimmune disorders.
Read more:
- MedImmune, Joslin Partner on Diabetes Research
- Registry to Track Psoriasis Drug Safety
- Biosimilar Shown Effective for Rheumatoid Arthritis
- FDA Approves Novartis Psoriasis Drug
- Autoimmune Therapy Developer Raises $23M in Early Funds
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.