10 June 2015. An early-stage clinical trial is recruiting patients to test the safety of a treatment for severe gout that harnesses the immune system, but still avoids unwanted immune responses. The trial is conducted by the biotechnology company Selecta Biosciences at two sites in the U.S.: Altoona, Pennsylvania and Dallas, Texas.
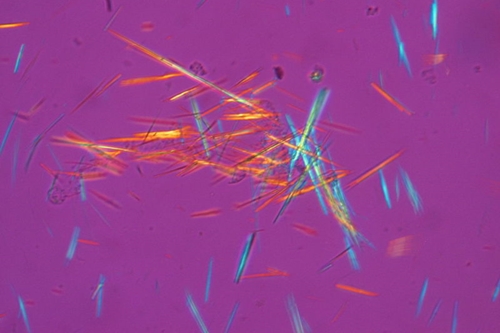
Gout is a complex form of arthritis, marked by sudden and severe episodes of pain, with tenderness and redness in the joints, particularly at the base of the big toe. Men tend to be affected more than women, but women become more susceptible to gout after menopause. Gout occurs when uric acid builds up and forms needle-like crystals called tophi in the joints or surrounding tissue, causing the pain and inflammation. The company cites data estimating gout affecting 4.7 million people in the U.S.
Uricase is an enzyme found in some animal species, but not humans, that oxidizes uric acid making it more soluble, so uric acid can be removed from the body. However, uricase also causes an immune system reaction, which sharply limits its use in natural form as a gout treatment.
Selecta Biosciences, in Watertown, Massachusetts, is developing a therapy for severe gout that it says prevents the undesired immune reactions. The biologic therapy, code-named SEL-212, adapts a form of uricase combined with polyethylene glycol, or Peg, a non-toxic natural polymer that does not cause an immune reaction and improves the actions of biologic drugs in the body. Selecta licensed this agent, called pegsiticase, a year ago from a Chinese biotechnology company.
SEL-212 delivers pegsiticase in Selecta’s synthetic vaccine particle technology. That technology generates biodegradable nanoparticles directing the immune system to prevent and suppress immune responses to specific antigens. Thus specific immune responses are blocked without compromising an individual’s entire immune system. The company says it tested the technology in preclinical proof-of-concept studies.
The clinical trial aims to to define safe and effective dosages for the treatments. Administrators are recruiting 25 individuals with high uric acid levels, who will receive a single dose of pegsiticase via intravenous infusion at varying levels. The trial will look primarily for any adverse reactions for 30 days following the infusion. However, the study is also measuring actions of pegsiticase in the body, including changes in uric acid levels and formation of anti-drug antibodies indicating an adverse immune reaction.
SEL-212 is Selecta’s lead therapy candidate. The company has other products in its pipeline, including gene therapies, designed to treat hemophilia, type 1 diabetes, celiac disease, and food allergies, in discovery and preclinical stages.
Read more:
- Trial Shows Immunotherapy Reduces Rheumatoid Arthritis
- Spin-Off Biotech Formed for Autoimmune Disorders
- Sanford-Burnham, Lilly Partnering on Immune Therapies
- Biotech, Research Institute Partner on Gene Therapies
- Lilly Acquiring Treatment for Autoimmune Disorders
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.