12 August 2015. Global Blood Therapeutics, a biotechnology company developing treatments for blood-related disorders, is raising $120 million in its initial public stock offering. The company, in South San Francisco, California and trading on the Nasdaq exchange under the symbol GBT, issued 6 million shares priced at $20.00. Shares closed today trading at $43.50, more than double the IPO price.
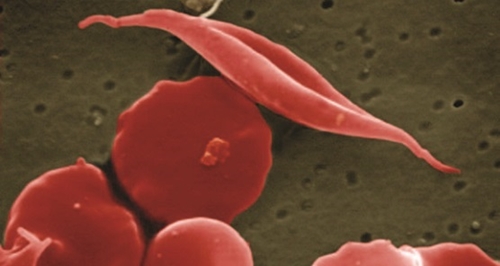
Global Blood Therapeutics, founded in 2012 as a spin-off enterprise from University of California in San Francisco, designs treatments for sickle cell disease and related disorders that aim to address the root causes of disease rather than manage disorders. Sickle cell disease is a genetic blood disorder affecting hemoglobin that delivers oxygen to cells in the body. People with sickle cell disease have hemoglobin molecules that cause blood cells to form into an atypical crescent or sickle shape.
That abnormal shape causes the blood cells to break down, lose flexibility, and accumulate in tiny capillaries, leading to anemia and periodic painful episodes, called vaso-occlusive crises. The disease is prevalent worldwide, and affects 70,000 to 80,000 people in the U.S., including about 1 in 500 people of African descent.
Global Blood Therapeutics’ technology aims to change the shape of blood proteins for treating sickle cell diseases and other genetic disorders. The company’s technology platform combines computational biology and modeling of proteins and ligands — signaling molecules that bind to proteins — with medicinal chemistry and empiric screening.
The lead product from the company, code-named GBT440, is designed as an oral preventative medication taken once a day. GBT440 is designed to block the process of hemoglobin in its de-oxygenated state to cause red blood cells to clump together and form into abnormal sickle shapes. Currently, two early and intermediate stage clinical trials are recruiting healthy volunteers and individuals with sickle cell disease to test the safety, chemical activity, and biological effects of GBT440.
Global Blood Therapeutics’ founders include three biomedical researchers from University of California in San Francisco — pharmaceutical chemist Matthew Jacobson, computational biologist Andrej Sali, and pharmacologist Jack Taunton. In addition to its scientific founders, the company was started by biotechnology entrepreneurs Charles Homcy and Craig Muir, with Third Rock Ventures partner Charles Homcy. Third Rock Ventures provided early financing for the company, as reported by Science & Enterprise.
Read more:
- Peanut Allergy Therapy Company Raises $160 Million in IPO
- Drug Delivery Biotech Raises $101.8 Million in IPO
- Rare Disease Therapy Company Raises $60 Million in IPO
- Cancer Biotech Raising $147 Million in IPO
- Aduro Biotech Raises $108 million in IPO
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.