24 September 2015. Intarcia Therapeutics, a developer of long-acting treatments for diabetes, is purchasing Phoundry Pharmaceuticals, a discoverer of engineered peptides for metabolic and other disorders. Phoundry — a spin-off company from GlaxoSmithKline in Research Triangle Park, North Carolina — is being acquired by Intarcia for an undisclosed amount of cash and stock.
Intarcia, based in Boston, is developing therapies for type 2 diabetes. Its lead product, code-named ITCA 650, is a system consisting of exenatide, a drug that acts like a hormone in the body known as incretin to help control blood sugar. Exenatide, like incretin, prompts the pancreas to release insulin, and at the same time prevents the hormone glucagon from stimulating the liver to release sugar into the blood stream. In addition, the drug helps slow the rate at which the stomach empties after a meal, keeping an individual feeling full longer, thus also keeping blood sugar from rising too quickly after eating.
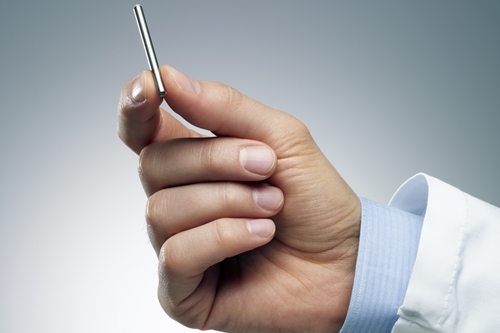
ITCA 650 also includes a delivery method for exenatide, where a pump the size of a matchstick is inserted under the skin to provide a continuous dose of the drug through osmosis for up to one year without replacement. Because the pump is inserted under the skin, notes Intarcia, a qualified clinician can quickly remove it if needed. Today, the use of exenatide and other drugs acting like incretin is limited by the need for people with type 2 diabetes to self-inject the drug twice a day, with nausea a common side effect.
Phoundry Pharmaceuticals discovers peptides, simple protein molecules optimized or enhanced with therapeutic properties. The company was founded earlier in 2015, by six former scientists with GlaxoSmithKline working on therapies for metabolic diseases, including experience with exenatide.
Among the optimized peptides being designed by Phoundry are those to extend the metabolic benefits and potential weight loss effects found with bariatric surgery. Intarcia president Kurt Graves says in a company statement, “Their team has a proven track record of producing medicines and they have worked for years to design optimized peptides to specifications that are a perfect fit for our targets and our mini-pump delivery systems.” The company indicates it plans to test two of Phoundry’s optimized peptides as enhancements to ITCA 650, targeting type 2 diabetes and obesity.
In March 2015, Intarcia licensed antibody technology from the biotechnology company Numab AG in Wädenswil, Switzerland. Numab’s technology identifies rare antibody fragments that act as building blocks for designing therapeutics that bind to antigens and stimulate an immune response. Intarcia says it plans to integrate Numab’s technology to test the addition of an antibody fragment to ITCA 650 also targeting type 2 diabetes and obesity.
In August 2015, as noted in Science & Enterprise, Intarcia reported initial results from a late-stage clinical trial showing more participants fitted with ITCA 650 reached their target blood glucose levels, measured as concentrations of glucose in hemoglobin, than those taking sitagliptin, a blood glucose control drug made by Merck. Study participants with ITCA 650 devices also reported an average weight loss of 2 kilograms (4.4 pounds) or more, a secondary objective of the study.
Read more:
- Sanofi, Google to Partner on Diabetes Care, Devices
- Glucose Control Implant Bests Diabetes Drug in Trial
- Mobile Diabetes Device Maker Lands $20M in Venture Funds
- MedImmune, Joslin Partner on Diabetes Research
- Type 2 Diabetes Technology Licensed in $1 Billion Deal
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.