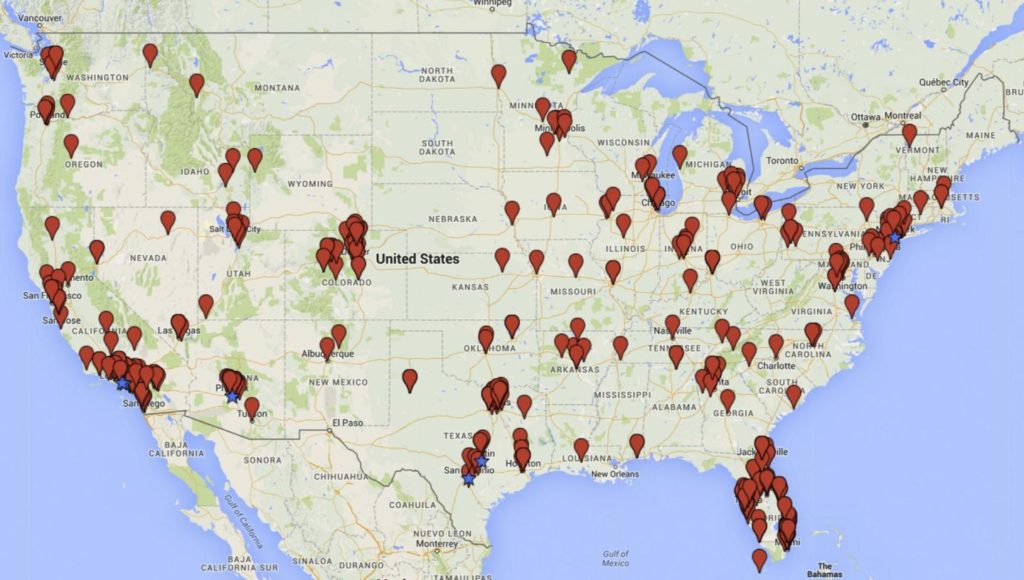
Map of continental U.S. with locations of stem cell clinics. Blue stars indicate hot-spot concentrations. Click on image to view full size. (Leigh Turner and Paul Knoepfler)
1 July 2016. A comprehensive Internet search finds nearly 600 clinics in the U.S. claiming to offer stem cell treatments, many having dubious scientific or medical validity. The analysis by bioethicist Leigh Turner and stem cell researcher Paul Knoepfler is published in yesterday’s issue of the journal Cell Stem Cell.
Turner at University of Minnesota and Knoepfler at University of California in Davis, sought to better understand the extent of medical treatments marketed in the U.S. that claim to based on therapeutic benefits of stem cells. Recent news reports, including a story just last week in the New York Times, tell about catastrophic health consequences of stem-cell tourism offered by clinics in Russia, China, and South America. Turner and Knoepfler indicate that similar problems may be developing much closer to home, inside the country’s borders.
The authors conducted systematic and comprehensive key-word Internet searches, on terms such as “stem cell treatment” and “stem cell therapy,” combined with text mining and content analyses of Web sites. Their searches revealed 351 businesses offering stem cell medical services through 570 clinics. Nearly 4 in 10 of these clinics (38%) are located in either Florida or California, with 18 clinics alone in Beverly Hills and another 12 in Los Angeles. Other concentrations of clinics are in New York City, San Antonio and Austin in Texas, and Scottsdale and Phoenix in Arizona.
Many businesses with stem cell services offer treatments for a wide range of conditions, but the two leading applications are for orthopedic and pain disorders. Many clinics offer anti-aging and cosmetic applications, including face lifts and breast augmentation based on stem cells. However, the researchers also found therapies for more serious disorders including spinal cord injuries, immunological, cardiac, pulmonary, ophthalmologic, and urological diseases.
In addition, the researchers found a number of marketing pitches for stem cell treatments aimed at parents and guardians of children with inherited disorders, and caregivers of individuals with neurodegenerative conditions, such as dementia. The team found 33 claims for treatments of muscular dystrophy, as well as 9 therapies for autism and cerebral palsy. Another 27 enterprises offer treatments for Alzheimer’s disease.
Most of the businesses offering stem cell services use autologous stem cells, those taken from the patient. About 6 in 10 companies derive stem cells from adipose or fat-storing tissue, while nearly half (48%) take stem cells from bone marrow. About 1 in 5 enterprises advertised allogenic stem cells, those from external sources, mainly amniotic materials or fluids, as well as placenta tissue, and umbilical cords.
Turner and Knoepfler say a legal or regulatory analysis of these services is outside the scope of their paper, but they conclude that their results raise concerns about safety and efficacy of these treatments as well as the ethics of raising false hopes and charging high prices for therapies with dubious marketing claims. The authors note FDA issued draft guidelines for regulating products based on stem cells in October 2015, and cited news reports the agency is preparing to crack down on clinics marketing phony cures.
Read more:
- More Device Approvals, Higher Risks in Europe than U.S.
- New Diabetic Eye Disease Drugs Shown Not Cost-Effective
- NIH Closes Two Clinical Facilities for Sterility Issues
- Wide Variation Found in Consumer Blood Tests
- Medical Industry Payment Recipients Identified
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.