12 Nov. 2019. A system with electronic sensors added to pills helps people known to skip taking medications stick with their drugs prescribed for hepatitis C. Findings from a clinical trial evaluating the DigiMeds system made by Proteus Digital Health in Redwood City, California, among patients with a history of non-adherence to drugs, were presented on Sunday, 10 November at a meeting of American Association for the Study of Liver Diseases in Boston.
Hepatitis C is a viral infection affecting the liver, with some 2.4 million living with the virus in the U.S., according to Centers for Disease Control and Prevention. The disease is transmitted through contact with infected blood, with intravenous drug users sharing needles among those at the highest risk of contracting the disease. The virus causes inflammation of the liver and can lead to cirrhosis or scarring and poor liver function over many years.
Because there are no symptoms early on, many people with hepatitis C infections do not get treatment until more serious complications occur. And even when treatment is started, many individuals with the virus are often at high risk of not adhering to their therapies, often due to difficult life circumstances associated with substance abuse, such as psychiatric disorders or alcoholism.
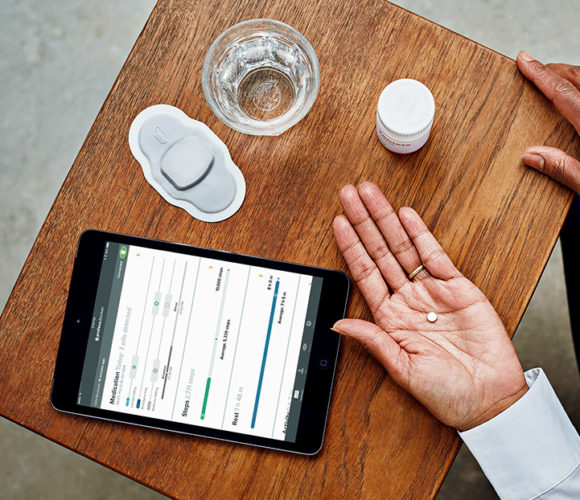
Proteus Digital Health offers what the company calls its digital medicines or DigiMeds program that adds a tiny electronic sensor to medications in a capsule to track adherence. The system, formerly called Discover, also has an electronic patch worn on the torso that reads signals from the sensor, a mobile app that reads data from the patch to record the individual’s taking of medications, and a web portal used by health care providers and caregivers to track patients’ adherence to treatments.
The clinical trial, led by Mark Sulkowski, a Johns Hopkins University medical school professor that studies management of viral hepatitis, recruited 288 individuals with hepatitis C at 18 clinics in the U.S. The study team was particularly interested in enrolling people with a history of medication non-adherence, and the trial’s sample reflected that profile: 52 percent of participants reported alcohol or substance abuse, while 23 percent had a serious psychiatric disorder. About one in five participants (19%) also had HIV infections. Most trial participants reported that their families earned less than $25,000 per year and nearly 1 in 10 participants said they were homeless.
In the trial, the Proteus DigiMeds sensor was added to direct acting antiviral medications, combinations of drugs taken daily to reduce hepatitis C viral loads. Participants were asked to report back to the clinics after 4 and 12 weeks to assess their sustained virologic responses, indicating the treatments were clearing their blood of hepatitis C, while the DigiMeds system tracked medication adherence.
Among the initial participants, 205 reported for the 4-week check and 217 for the 12-week check. The study team says drop-outs occurred for a variety of reasons, including 30 participants who did not respond to follow-ups and 6 individuals reporting adverse effects, of which 3 rash cases were attributed to the treatments. Of those reporting after 4 weeks, all were found with sustained virologic responses, and after 12 weeks nearly all — 216 of 217 — likewise had sustained virologic responses. At both points, adherence to prescribed medications averaged 93 percent among participants.
“This is encouraging data showing how DigiMeds can make a significant impact on curing patients at high risk of non-adherence,” says Andrew Thompson, CEO of Proteus Digital Health in a company statement. “As we pursue the use of DigiMeds across therapeutic areas, we believe we will continue to see improved patient outcomes.”
More from Science & Enterprise:
- Partnership to Assess Digital Gut Disease Treatments
- Trial Testing Mobile Telemedicine for Skin Diseases
- Trial to Test App Alerting for Dementia-Linked Drugs
- FDA Clears Wearable App Device for Migraine
- Sensors, Algorithm Personalize Parkinson’s Therapy
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.