2 Jan. 2020. A system for automatically measuring blood glucose levels and dispensing insulin for people with type 1 diabetes is authorized by the Food and Drug Administration. The system uses an insulin pump made by Tandem Diabetes Care and glucose monitor by Dexcom Inc., both in in San Diego, controlled by software based on research at University of Virginia in Charlottesville.
Type 1 diabetes is an inherited autoimmune disorder where islet cells, also known as beta cells, in the pancreas do not produce insulin. Type 1 diabetes is diagnosed primarily in children or young adults, where the immune system is tricked into attacking healthy cells and tissue as if they were foreign invaders, in this case, insulin-producing islet cells. From 5 to 10 percent of people diabetes have the type 1 form, estimated at 1.25 million in the U.S.
People with type 1 diabetes need to constantly test their blood to keep glucose or sugar levels in a safe range. Up to recently, those tests required testing a tiny blood sample from sticking a needle into a finger. More recently, glucose monitors attached to the skin came to market that constantly monitor blood glucose levels, along with insulin pumps that deliver the precise dose of insulin. These devices, however, still need human coordination and control to keep blood glucose levels within a safe range.
Missing from the overall system is a way to automatically control and coordinate both devices. The Center for Diabetes Technology at University of Virginia conducts research on analytics for understanding and managing diabetes, and with colleagues at Harvard University, developed algorithms for measuring blood glucose and calculating insulin requirements. Those algorithms were translated into software, licensed to the spin-off enterprise TypeZero Technologies in Charlottesville, for commercial development. In 2018, Dexcom acquired TypeZero Technologies, incorporating the software into Dexcom’s systems.
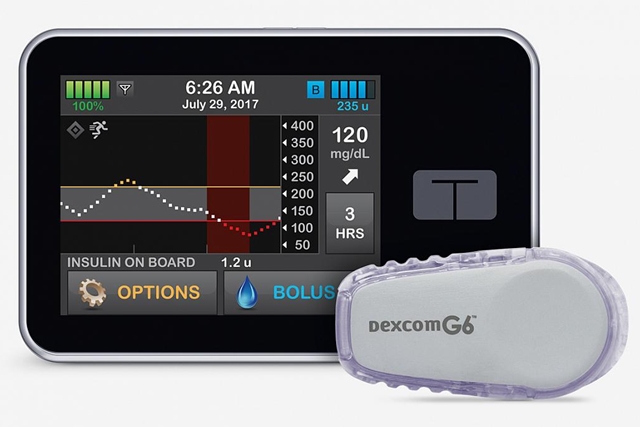
The complete closed-loop system combines the t:slim X2 insulin pump made by Tandem Diabetes Care with the Dexcom G6 integrated continuous glucose monitor, both managed by Control-IQ software from the original algorithms developed at University of Virginia. This so-called artificial pancreas uses signals from the glucose monitor to drive the timing and quantity of insulin released from the pump, without any action by the wearer.
“This is a new-generation interoperable automated glucose control system,” says Boris Kovatchev, Center for Diabetes Technology director in a university statement, “which allows seamless integration of a continuous glucose sensor, insulin pump, and a smart control algorithm.” Kovatchev is one of the team leaders that developed the original algorithms and software.
In a clinical trial published in the New England Journal of Medicine and reported by Science & Enterprise in October 2019, participants were randomly assigned to wear the closed-loop system or the sensor-augmented insulin pump. Results show participants wearing the closed loop system spend more hours per day in the safe-target range than sensor-augmented pump wearers. The percentage of safe-target time at night — midnight to 6:00 am — is also higher for closed-loop system participants. In addition, the percentage of time spent in the safe-target range increased during the six-month trial for closed-loop system wearers, while sensor-augmented pump wearers stayed the same.
FDA cleared the closed-loop system in mid-December, for people in the U.S. with type 1 diabetes needing fine-tuned insulin deliveries based on day-to-day needs, as well as personal history. The agency noted the device is the first closed-loop system, but also integrates devices from different manufacturers, a step FDA hopes leads to more interoperability in these systems.
More from Science & Enterprise:
- Device Offers Real-Time Monitoring of Swallowing Problems
- Retail Clinics Offer A.I.-Enabled Eye Diagnostics
- Peanut Allergy Patch Under FDA Review
- FDA Clears Wearable App Device for Migraine
- Insulin Delivered by Capsule, That’s the Good News
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.