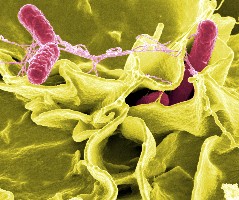
Salmonella typhimurium, in red, invading cultured human cells. (Rocky Mountain Laboratories, NIAID, NIH)
Agilent Technologies Inc. in Santa Clara, California, a developer of instrumentation for chemical and life science analysis, unveiled its agreement with the U.S. Food and Drug Administration to develop new tests for the agency’s regulation of food. One set of tests for FDA will identify salmonella more precisely, while a different test suite will use DNA to identify species of fish, particularly after processing.
The salmonella tests are expected to help FDA more precisely identify potential sub-types of salmonella in food. Knowing the sub-type of salmonella can help authorities more quickly identify the source of the pathogen and limit the number of victims. Agilent says it anticipates adapting its mass spectrometry technologies to devise this solution.
The fish species tests will be developed, says the company, to meet FDA’s need for a fast, simple method of determining the species of fish used in processed fish products, where identifying features such as the head, tail, and skin are removed. This type of test could detect intentional mislabeling, particularly of imported products, to avoid tariffs and import restrictions, or fraudulent labeling where a less expensive species of fish is sold as a more costly species.
For the fish species tests, Agilent expects to adapt its microfluidics-based analysis methods that use lab-on-a-chip technologies to identify DNA from small substance samples. On this part of the project, Agilent plans to partner with the Campden BRI Laboratory in the U.K. that specializes in food analysis and testing.
Read more: Agilent, Singapore Institute Launch Drug Screening Center
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.