Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on March 25th, 2022  (Victoria Borodinova, Pixabay. https://pixabay.com/photos/cover-your-face-with-your-hands-hide-6599123/) 25 Mar. 2022. Findings from a clinical trial show a rapidly disintegrating cannabidiol tablet reduces pain with few adverse effects in patients having shoulder surgery. Results of the trial conducted at medical centers in New York and Jacksonville, Florida are scheduled for presentation today at a meeting of the American Academy of Orthopaedic Surgeons in Chicago.
Health authorities continue to search for non-opioid alternatives for treating acute pain, such as from trauma or surgery. Opioid drugs are still prescribed for acute pain in many cases, which can lead to dependence or abuse. In the U.S., abuse of opioid pain drugs continues at rates reaching emergency levels, along with heroin and fentanyl sold on the street. Last month, Science & Enterprise reported on new Food and Drug Administration regulatory guidance for review of non-opioid treatments for acute pain.
The clinical study tested a pain treatment made from cannabidiol or CBD, a compound derived from the Cannabis sativa plant, but does not produce an emotional high associated with marijuana, also a derivative of cannabis. National Library of Medicine in the U.S. says some 80 chemicals, known as cannabinoids, are associated with these plants. One of those chemicals, CBD, a non-intoxicating derivative, makes up about 40 percent of cannabis extracts and has been studied extensively for a range of disorders.
The pain treatment in this case is Oravexx, developed Orcosa Inc. in Ewing, New Jersey. Oravexx contains a CBD compound formulated into a tablet designed to quickly disintegrate while in the mouth. As the tablet disintegrates, says Orcosa, its active ingredients are absorbed by tissue in the cheek in about three seconds. People taking Oravexx or other medications using this technology do not need to drink water or swallow a pill.
Less severe pain after one day
The early-stage clinical trial enrolled 99 adult patients receiving arthroscopic rotator cuff repair, a common injury of shoulder muscles, particularly among athletes. All participants first received a low dose of the opioid pain-killer Percocet immediately after surgery, as well as instructions to wean themselves from the drug as quickly as possible. Participants then received Oravexx in 25 or 50 milligram doses or an oral-disintegrating placebo, taken three times a day for 14 days.
The study team, led by NYU orthopedic surgery professor Michael Alaia, looked primarily at participants’ responses to a standard visual scale of pain experience, as well as reports of nausea, both for 14 days. Participants also reported any other use of opioids or CBD over that time, as well as satisfaction with their treatment.
Results show patients receiving Oravexx rate their pain as 23 percent less severe than placebo recipients, one day after surgery. Oravexx recipients also report 22 to 25 percent greater satisfaction with their treatment than placebo recipients in the first two days following surgery. In addition, Oravexx recipients in 50 milligram doses report less pain and more satisfaction with their treatment than the 25 milligram dose recipients. And researchers say Oravexx participants report no major adverse effects.
“There is an urgent need for viable alternatives for pain management,” says Alaia in an NYU Health statement, “and our study presents this form of CBD as a promising tool after arthroscopic rotator cuff repair.” Alaia adds, “It could be a new, inexpensive approach for delivering pain relief, and without the side effects of anti-inflammatory drugs like NSAIDs and addiction risks linked to opiates.”
In an Orcosa statement, company founder and CEO Mark Ridall says the clinical trial, “lays the foundation for several upcoming phase 2 (mid-stage) studies in the treatment of acute and chronic pain utilizing Oravexx.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on March 24th, 2022  Vaccine patch system in use (Vaxxas Pty Ltd) 24 Mar. 2022. A company developing a needle patch for vaccine delivery is acquiring the rights to a synthetic SARS-Cov-2 spike protein for its Covid-19 vaccine patch. Vaxxas in Cambridge, Massachusetts is licensing a protein known as HexaPro, developed by biomedical and engineering researchers at University of Texas in Austin.
HexaPro is the work of a research team led by molecular biologist Jason McLellan, with molecular biologist Ilya Finkelstein and chemical engineering professor Jennifer Maynard, all at UT-Austin. Most Covid-19 vaccines target the protein covering spikes on the SARS-CoV-2 virus, which binds with angiotensin-converting enzyme 2 or ACE2 receptors in cells to start infections. Proteins in their wild-type form are unstable and break down easily, and have a short shelf life. Thus, vaccine developers need synthetic proteins like HexaPro with a similar chemistry to generate an immune response, but last a longer time and need no special handling such as deep-freezing.
The UT-Austin researchers also designed HexaPro to be produced inexpensively, with the vaccine manufactured in eggs as most influenza vaccines today. In June 2021, Science & Enterprise reported on a preclinical test of HexaPro with lab mice reported on a preprint server, but later peer-reviewed and appearing in October in the journal Science Advances. In the study, researchers administered HexaPro with a high-density micro-array needle patch, or HD-MAP, where one dose of the vaccine resulted in neutralizing antibodies against the original SARS-CoV-2 as well as alpha and beta variants. And the vaccine delivered through the patch protected healthy mice against infection-related disease, but also better than vaccines delivered with a syringe.
Clinical trial planned later this year
Vaxxas is a developer of high-density microneedle patches for vaccine delivery. Each patch is made of ultra-short (0.25 mm) microneedles coated with a dry form of the vaccine. The tiny patch is packaged in a single-use spring-loaded applicator that pushes the microneedles into the skin. Once under the skin, the vaccine contents enter capillaries and travel to lymph nodes where they trigger an immune response. Vaxxas offered its microneedle patch to HexaPro researchers for the team’s preclinical studies published last year.
UT-Austin is providing Vaxxas with an exclusive license for HexaPro delivered via a patch device. Financial terms of the agreement are not disclosed. Vaxxas says it also acquired a related background technology from National Institutes of Health. The company says the license will enable Vaxxas to begin clinical trials of the HexaPro patch, with an early-stage clinical study planned for late this year.
“The compelling preclinical results published in Science Advances,” says Vaxxas CEO David Hoey in a company statement released through BusinessWire, “established the potential superiority of the HD-MAP/HexaPro Covid-19 vaccine compared to delivery using the traditional needle and syringe. We can now rapidly advance our clinical trials with our next-generation Covid-19 vaccine patch.”
“Just as the virus has changed and evolved,” notes McLellan, “our vaccines need to keep up with the latest challenges, too, and a key challenge now is vaccinating the world. It’s exciting to see HexaPro on the cusp of entering clinical trials with a patch-based technology that could be a real game-changer for parts of the globe where access to vaccines has been limited so far.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on March 23rd, 2022  Gregory Verdine (Harvard University) 23 Mar. 2022. A biotechnology company discovering protein therapies in fungi is collaborating with drug maker GlaxoSmithKline to find new small molecule drugs. GSK is also part of a consortium raising $175 million in venture funds, investing in LifeMine Therapeutics Inc. in Cambridge, Massachusetts.
LifeMine Therapeutics discovers and develops small molecule, or low molecular weight drugs from fungal genomes, encoded and linked to human proteins with computational techniques. The company says it uses machine learning algorithms to find indicators or avatars in fungi genomics associated with human genes and proteins, and discover related small molecule drugs with chemical properties addressing desired targets and outcomes. And LifeMine says it also uses informatics to streamline its lead optimization process.
The five year-old company was founded by Gregory Verdine, a professor of chemistry at Harvard University, as well as serial entrepreneur and venture investor, with Richard Klausner, founder of Juno Therapeutics and Grail, and WeiQing Zhou, a co-founder with Verdine of other businesses. The deal with GSK is LifeMine’s first collaboration to advance new drugs from its platform.
“This is a transformative collaboration for LifeMine, and marks the first such agreement in genomic drug discovery from fungi, nature’s virtuoso medicinal chemists,” says Verdine in a LifeMine Therapeutics statement released through BusinessWire. Verdine is the company’s president, CEO, and chief scientist.
Third venture financing round
The agreement calls for LifeMine Therapeutics and GSK to partner on discovery of small molecule drugs addressing up to three undisclosed targets. The companies will also collaborate on preclinical work, sharing costs up to filing for an investigational new drug application, or IND, in effect a request to the Food and Drug Administration to begin clinical trials. GSK will be responsible for further drug development and commercialization.
LifeMine is receiving an initial payment from GSK of $70 million divided between cash and equity investment as part of its new venture round. The company is also eligible for undisclosed discovery, development, and commercial milestone payments for each product from the collaboration, as well as royalties on sales revenues.
LifeMine Therapeutics is raising $175 million in its third venture round led by Fidelity Management & Research Company, with participation by Invus, 3W Partners Capital, and GSK all new investors. Also taking part are current investors GV (previously Google Ventures), Arch Venture Partners, Blue Pool Capital, and MRL Ventures Fund. The company expects to use the proceeds to further refine its technology called Avatar-Rx and advance its lead programs. LifeMine previously raised $105 million in its first two venture rounds, in Sept. 2017 and Jan. 2021.
“This financing will allow us to further scale our Avatar-Rx platform,” notes Verdine, “and advance our lead programs targeting some of the most elusive and high value genetically validated drivers of cancer.” He adds that the new financing and collaboration with GSK, “leaves us well-positioned to rapidly advance multiple high-impact precision medicines to solve some of the most intractable challenges facing modern medicine.”
More from Science and Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on March 22nd, 2022  Printed lymph node organoid (Prellis Biologics Inc.) 22 Mar. 2022. A biotechnology enterprise plans to discover synthetic antibodies with 3-D printed lymph node tissue for future licensing to a pharma company. Financial details of the agreement between Prellis Biologics Inc. in Hayward, California and global pharmaceutical company Sanofi were not disclosed.
Prellis Biologics is a tissue engineering and antibody discovery company that creates three-dimensional cells and tissue samples with a bioprinting technology. One of the company’s main 3-D printed tissue products is functioning lymph node tissue called organoids. Prellis Biologics engineers its lymph node organoids to respond to antigens in much the same way as human lymph nodes, to produce a wide range of synthetic antibodies meeting specific requirements and properties in as little as three weeks. The company says its lymph node organoids are used for therapies, treatment screening, and vaccine testing.
Prellis Biologics calls its lymph node organoid technology an external human immune system, or Exis. “The Exis platform,” says Prellis’s founder and CEO Melanie Matheu in a company statement released through BusinessWire, “provides an unparalleled ability to recreate lymph node organoids in vitro. This powerful technology enables investigation of human B- and T-cell responses for a variety of applications, including antibody discovery and therapeutic immunogenicity.”
Laser holograms to print extracellular matrix
In their agreement, Prellis Biologics and Sanofi will collaborate on producing human antibodies from lymph node organoids addressing a specified, but undisclosed target. Sanofi gains an option to license, develop, and commercialize antibodies generated by the collaboration. In return, Prellis receives an initial payment, but the the payment amount is not disclosed.
Science & Enterprise first reported on Prellis Biologics’ seed funding and incubation in Sept. 2017. The company’s tissue engineering technology is based on Matheu’s work as a graduate student at University of California in Irvine and postdoctoral researcher at University of California in San Francisco. In her doctoral research, Matheu studied the laser-based imaging processes underlying the company’s technology, including the application of those processes to print fine blood vessels, called microvasculature, permeating human tissue and organs.
Prellis uses laser holograms that put down fine layers of extracellular matrix, secretions from cells that provide structural and biochemical support, already containing cells at near instantaneous speeds. Unlike most current tissue engineering methods, the Prellis process, according to the company, does not require previous cell seeding or additional culturing to produce tissue matrix. As a result, it achieves high printing speeds, which are critical for tissue production since densely packed cells will die in less than 30 minutes unless oxygen and nutrients can be supplied immediately with blood carried through capillaries.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on March 22nd, 2022 – Contributed content –
 (TheDigitalWay, Pixabay) 22 Mar. 2022. These days, it is all too simple to find yourself in a state of financial distress. First, you purchase something with the intention of paying it off on payday, only to discover that you really need the money now. Getting an overdraft is simple when you persuade yourself that you can easily pay the expenses. Unfortunately, we’ve been conditioned to believe that debt is a normal part of life, and this article will teach you how to deal with it when it gets out of control.
As a first and foremost consideration, being more thrifty with the resources you have at your disposal is one of the most beneficial things you can do for your wallet, both in the long and short term. Try a cheaper brand of a product and compare it to the more expensive ones. Snag the bargains on the supermarket’s clearance racks. Because they have to sell the item by that date on their label, you normally have a few days to use up any food that has been purchased beyond that date. Of course, in this case, you should also use caution and good judgement. To save money on new furniture while redesigning a room, why not upcycle an existing piece and perform any required repairs instead of paying for a brand new one? Practicing a more frugal lifestyle can benefit you both now and in the future.
If you’ve got a lot of debt, you may want to think about obtaining a debt consolidation loan. With this plan, you’ll be able to pay off all of your debts over a period of time. Consolidation loans are particularly excellent if you have interest-bearing debt since you may pay it all off at once and avoid any further interest accrual. Visit and compare credit companies online to learn more and see whether it’s a possibility for you. Even if you have a low credit score, it doesn’t mean you can’t get your finances in order.
Getting a second job or working overtime at your current employment might help you save money for debt repayment. Understandably, you don’t want to keep working two jobs, but paying off your bills and getting back on track is worth it.
Talking to your creditors to arrange a more reasonable repayment schedule is another option you should explore. If you ask about, you’ll be delighted at how accommodating businesses can be and how much of your debt they are willing to forgive in an effort to lend you a hand. The fact that you’ve explained your circumstances and have set up even a small monthly payment will demonstrate to them that you’re making every effort to pay off your debt.
Remember to spend wisely and follow these strategies to get out of debt and enjoy a debt-free life.
* * *
By Alan, on March 21st, 2022  (Heung Soon, Pixabay, https://pixabay.com/photos/tablets-medicine-supplement-vitamin-5620566/) 21 Mar. 2022. Findings from research with older lab mice show an oral drug addressing immune deficiencies protects the mice from death by SARS-CoV-2 infections. An unedited abstract with early results of the study, conducted by a team from University of Iowa in Iowa City and biotechnology company BioAge Labs in Richmond, California appears in today’s issue of the journal Nature.
BioAge Labs discovers and develops therapies for disorders affecting older people, using techniques from systems biology and artificial intelligence to identify promising drug targets. The company says it maintains large data sets from blood samples collected over a span of 45 years, connecting molecular variables with medical outcomes over that period. BioAge says these data collections make it possible to identify key drivers of pathology in old age with computational techniques, verified by preclinical studies with lab animals.
One of BioAge Labs’ lead products, code-named BGE-175, addresses the prostaglandin pathway in the immune system. This pathway, says the company, is part of the immune response to infections, including from SARS-CoV-2 viruses responsible for Covid-19, and particularly in older individuals. Prostaglandins are hormones produced in response to respiratory infections, as well as asthma. BioAge says BGE-175 blocks signaling from PGD2 DP1 protein receptors that stimulate the prostaglandin pathway. As a result, BGE-175 promotes dendritic cells in the immune system and maintains neutrophils, white blood cells that help fight infections.
90 percent of treated mice survived
Researchers led by Iowa microbiologist Stanley Perlman and medical school professor Paul McCray tested BGE-175 in mice reaching the equivalent of middle age. The animals were randomly selected to received daily BGE-175 treatments or go untreated, two days after receiving a lethal dose of SARS-CoV-2 virus. BioAge Labs says 90 percent of mice treated with BGE-175 survived their infections, while untreated mice died.
“We are encouraged by the study findings” says BioAge Labs co-founder and CEO Kristen Fortney in a company statement released through BusinessWire, ”and our preclinical data showing BGE-175’s potential to address multiple diseases, including Covid-19, pandemic influenza, and beyond, for which poor outcomes are driven by immune aging.” Fortney is also a co-author of the Nature paper.
“Our findings,” adds Perlman, “clearly show that the therapeutic target of BGE-175 plays a key role in making the aged lung environment conducive for optimal immune function and thereby counters immune aging. The drug’s protective effect in mice strongly supports the idea that BGE-175 corrects age-related declines in immunity, providing a strong rationale for testing in older patients who are hospitalized with Covid-19.”
BioAge is testing BGE-175 in a clinical trial as a treatment for Covid-19 infections in older patients. The mid-stage trial is enrolling 312 participants hospitalized with Covid-19 infections, age 50 and over, at 25 sites in the U.S., Argentina, and Brazil. Participants are randomly assigned to receive a daily BGE-175 or placebo tablet for 14 days, with participants tracked for up to two months. The study team is looking mainly at rates of respiratory failure or death among participants, but also other indicators of lung and overall health, as well as signs of adverse effects.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on March 19th, 2022 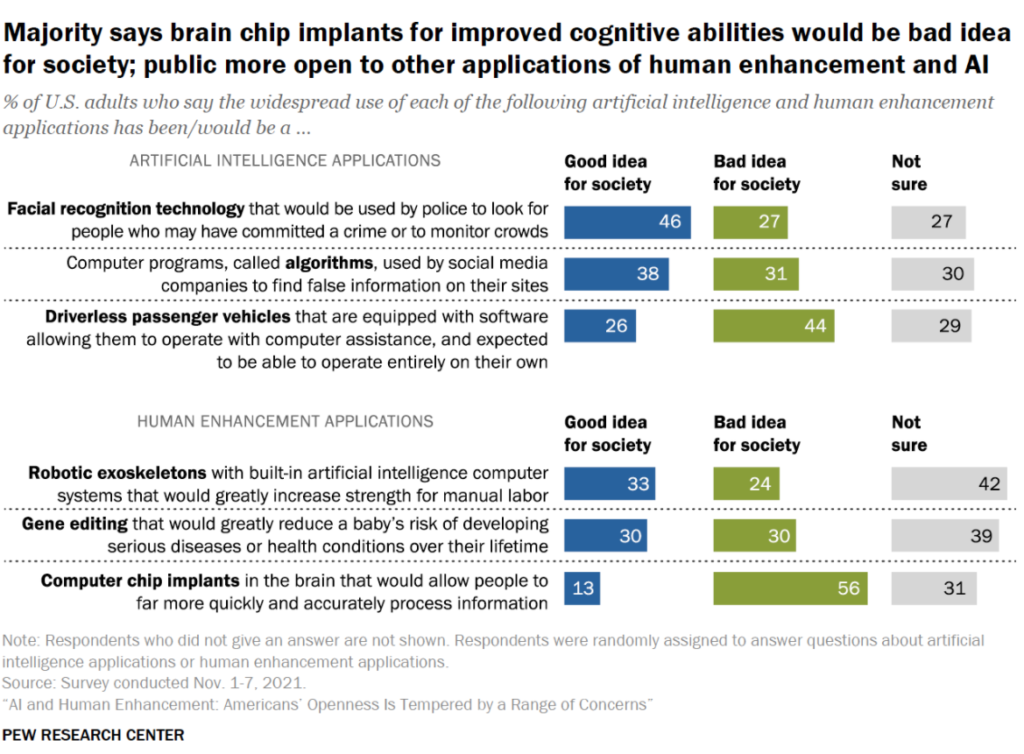 Click on image for full-size view (Pew Research Center) 19 Mar. 2022. U.S. residents are largely divided in their views of advances in artificial intelligence and genomics that they may experience now or in the future. Pew Research Center reported the findings this week from surveys the group conducted in November 2021.
The Pew researchers asked respondents if six new innovations in science and technology are a good or bad idea for society. In three of the examples — facial recognition for crime control, artificial intelligence algorithms in social media, and robotic exoskeletons — do more people believe these innovations are a good rather than a bad idea for society, but none draw majority support.
On gene editing, the U.S. public is evenly divided, with 30 percent each saying it’s a good or bad idea. And for chip implants in the brain to improve cognitive abilities and driverless vehicles, more respondents believe they’re bad rather than good ideas for society, with a majority (56%) believing brain chip implants are a bad idea. From about a quarter to four in 10 respondents, 27 to 42 percent, are not sure about the value of any of these advances.
On other questions, survey participants indicate more caution and skepticism than support for these technologies, with equal numbers — about three to four in 10 — believing gene editing and robotic exoskeletons will make life better as keeping life about the same as now. About a third of respondents (34%) say facial recognition will make police work more fair, while four in 10 (40%) say it won’t make much of a difference.
About four in 10 (39%) also believe driverless vehicles will decrease the number of people killed or injured in accidents, while three in 10 (31%) says it won’t make much difference. About a quarter (27%) believe the technology will increase accidents.
Pew Research Center conducted the survey 1 to 7 Nov. 2021, with 10,260 adult participants in its American Trends Panel representing a cross-section of the U.S. public. Participants completed online surveys on their own or with tablets furnished by the research team.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on March 18th, 2022  (Gerd Altmann, Pixabay) 18 Mar. 2022. A precision medicine research program at National Institutes of Health is releasing to scientists data for nearly 100,000 whole genome sequences. The All of Us initiative at NIH that collects the data says about half of the sequences represent racial and ethnic groups historically left out of genetic research.
The All of Us project seeks to gain better insights into biological, environmental, and behavioral factors that influence an individual’s health, taking into account a person’s lifestyle as well as their specific molecular makeup. All of Us is taking advantage of the wider availability and lower cost of genomic sequencing as well as expansion in the use of electronic medical records. As reported by Science & Enterprise in Jan. 2019, the initiative is recruiting one million participants to provide a wide range of personal health data, including from fitness devices.
The initiative is making the anonymous whole genome data available through its public researcher workbench. Whole genome sequencing, as the name implies, analyzes an individual’s almost entire genetic code. Most commercial genetic testing services sequence parts of the genome associated with ancestry or known diseases, called genotyping arrays. The research workbench, says NIH, also offers genotyping array data for some 165,000 individuals.
Supported by academic and industry labs
In addition, says NIH, the research workbench provides access to individuals’ electronic medical records, also with personal identifying information removed, to help researchers associate genomic factors with health outcomes. And the portal gives access to data from the American Community Survey, compiled by the U.S. Census Bureau. to associate health data with conditions in cities and towns.
Perhaps the most significant contribution of the new data release, says NIH, is the data’s ethnic and racial diversity, with about 50 percent of the genomes from people identifying as members of groups historically underrepresented in genetic research. “Until now, over 90 percent of participants from large genomics studies have been of European descent,” says All of Us CEO Josh Denny in an NIH statement. “The lack of diversity in research has hindered scientific discovery.” Denny adds that “Over time, as we expand our data and add new tools, this dataset will become an indispensable resource for health research.”
The All of Us program, including the research workbench, is supported by partners in academic and industry labs. The research workbench is managed by Vanderbilt University, with help from the Broad Institute of MIT and Harvard, and Verily Life Sciences, a division of Alphabet, the parent company of Google. The health technology company Color, in San Francisco, provides genetic analysis services for individuals providing specimens to All of Us.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on March 17th, 2022  (Sam Moqadam, Unsplash) 17 Mar. 2022. A clinical trial is evaluating data gleaned from wearable devices for measuring individual physiological reactions to Covid-19 vaccines. The trial’s sponsor, physIQ in Chicago, says enrollment is now complete for the study, conducted with contract research organization CellCarta in Montreal, Quebec, Canada.
The trial seeks to track immune system reactions by individuals receiving Covid-19 vaccines, with a combination of digital data captured by wearable devices and blood tests. physIQ generates health care analytics from biosensor data captured continuously with wearable devices including smart watches. The company says its analytics use machine learning algorithms that capture an individual’s vital signs from wearables, then provide a personalized report based on data from that person.
Food and Drug Administration, says physIQ, cleared the company’s remote monitoring algorithm to improve the accuracy of its assessments, as well as the company’s cloud-based analytics for heart rate, heart rate variability, atrial fibrillation detection, respiration rate, and personalized physiology change detection. Since the start of the Covid-19 pandemic, physIQ says it turned its capabilities to remote patient monitoring to help develop new vaccines and treatments. Science & Enterprise reported in May 2021 on an NIH-funded evaluation of the company’s health analytics to track physiological markers making up an index of clinical decomposition, as an early indicator of health decline.
Correlate physiological reactions to immune responses
CellCarta is a contract research organization specializing in precision medicine studies tracking biomarkers, or molecular indicators, of disease in individuals. The company says it can monitor genomics and analyze tissue samples worldwide, as well as provide detailed analytics of immune responses by clinical trial participants, including antibody and T-cell production.
The clinical trial is enrolling 130 adults and children in Chicago and Montreal not yet vaccinated against the SARS-CoV-2 virus, but with plans to receive a vaccine. Participants will provide health data from wearable devices such as heart rate and skin temperature, as well as measures of sleep and activity, before and after vaccination. In addition, about 30 participants will provide blood samples before and after vaccination to measure production of antibodies and T-cells in their immune responses to the vaccines.
The study team plans to correlate data from wearable devices to blood samples to find associations between vaccine-induced reactions, including adverse effects, to immune responses.Those data include detailed measures of physiological indicators, such as heart rate and skin temperature from before to after vaccination, changes in individual activity and sleep routines, and interactions of these data as well as deviations from expected results.
“Until now, there has never been a study that has looked so closely at individual differences in immune response to vaccines and their relationship to physiologic changes,” says Steven Steinhubl, physIQ’s chief medical officer in a statement. “Our goal is to provide tools that help accelerate the therapeutic development of personalized vaccine regimens by looking at the full immune response. This is important because we know there are unique differences in how people react to all vaccines.” Steinhubl, the study’s lead investigator, adds, “This near real-time capture of patient data potentially allows us to rapidly correlate physiological changes to immune responses and minimize possible adverse reactions early.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on March 16th, 2022  (Gerd Altmann, Pixabay) 16 Mar. 2022. A company creating a networked community defibrillator device for people suffering cardiac arrest is raising $22 million in its first venture round. Avive Solutions Inc., a five year-old enterprise in San Francisco, plans to offer a wireless, connected automated external defibrillator, or AED, to speed responses by health care workers and bystanders.
Avive Solutions aims to put life-saving AED technology in more hands throughout communities to help people experiencing sudden cardiac arrest in homes, offices, and elsewhere. Sudden cardiac arrest occurs when the heart stops beating, and blood stops flowing to the brain and other organs. Death can occur if not treated within minutes. A defibrillator helps restore a normal heartbeat by sending an electric pulse or shock into the heart. AED devices are appearing in more public places to treat people suffering from sudden cardiac arrest, with instructions for use by untrained bystanders.
Avive Solutions designed its device as a stand-alone AED, but it also connects to emergency response networks. When installed in workplaces, schools, churches, or gyms, the device gives instructions for bystander use, but also connects to 9-1-1 to alert first responders, and send incident and location data to speed response. The Avive Solutions AED can be installed in homes as well, and used to assist neighbors suffering cardiac arrest.
AED treatment within four minutes
According to Avive Solutions, the Food and Drug Administration is reviewing the device and has not yet authorized it for sale in the U.S. Nonetheless, the company is recruiting communities to take part in a program called 4-Minute City, to put enough AED devices throughout their populations so no person experiencing cardiac arrest is more than four minutes away from a defibrillator. According to the company, average emergency medical response times are eight to 12 minutes, increasing the risk of death from cardiac arrest. So far, says Avive Solutions, Jackson, Tennessee and Cumberland County, Pennsylvania are taking part in the program.
Company co-founders Rory Beyer and Moseley Andrews first developed their connected AED technology as undergraduate engineering students at MIT, joined by co-founder Sameer Jafri who also leads the Saving Hearts Foundation, an advocacy group for sudden cardiac arrest. Jafri is Avive Solutions’ CEO, while Beyer and Andrews are chief operating and chief technology officers respectively.
“Avive is changing the paradigm of response to cardiac arrest emergencies through our connected platform,” says Jafri in a company statement released through Cision. “By getting lifesaving automated external defibrillator, AED, technology in the hands of bystanders who can provide immediate help when and where it’s needed, and facilitating closer collaboration with emergency responders and health care providers, we believe our vision for a more streamlined system of care can greatly improve the odds of survival.”
Avive Solutions is raising $22 million in its first full venture founding round, led by Questa Capital, Catalyst Health Ventures, and earlier investor Laerdal Million Lives Fund. Other participants in the round are not disclosed. According to Crunchbase, the company raised $11.5 million in three installments between Dec. 2018 and June 2020.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|











 RSS - Posts
RSS - Posts
You must be logged in to post a comment.