Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on September 30th, 2023 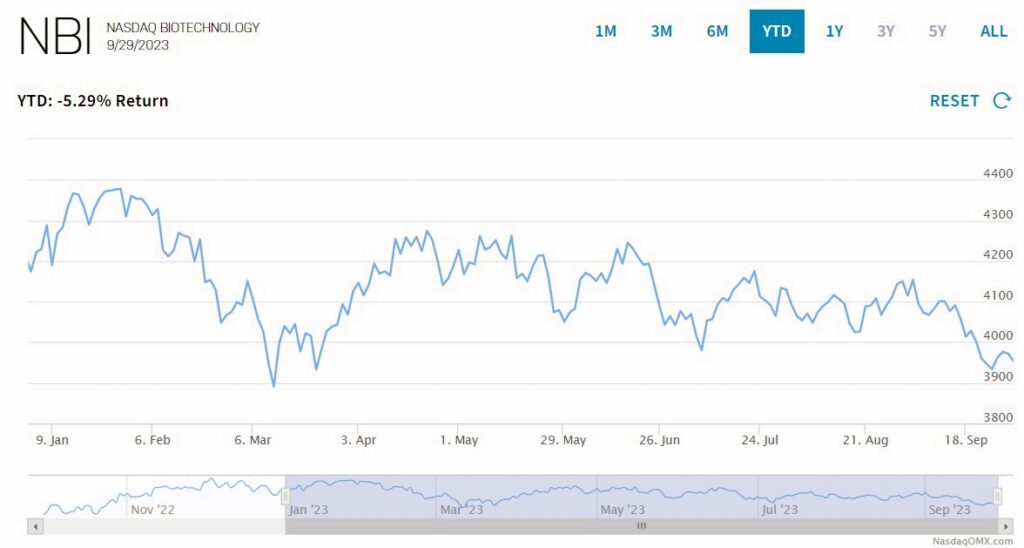 Click on image for full-size view (Nasdaq) 30 Sept. 2023. The Nasdaq Biotechnology Index, an indicator of investor sentiment in an industry based on science, continued heading down during the third quarter of 2023. NBI closed yesterday at 3,954, a decline of 5.3 percent since the start of trading on 3 Jan. 2023, and down 2.5 percent since the beginning of July.
The index, compiled with share prices of 262 biotechnology and pharmaceutical companies listed on the Nasdaq exchange, began rising during most of July, closing at 4,175 on 21 July, about the same as its price on the first day of trading this year. Then NBI fluctuated between 4,000 and 4,150 through the rest of July and August, only to decline through September to yesterday’s closing price below 4,000. The index’s price fluctuations showed a similar pattern in the second quarter of this year.
Despite the downward trend this year, NBI is still ahead by 4.9 percent of its close at the end of Sept. 2022. By comparison, however, the Nasdaq Composite Index, an indicator of investment sentiment in the technology sector overall, is up by 21.4 percent since the beginning of 2023 and 20 percent over the past 12 months.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 29th, 2023  Colorized scanning electron micrograph of a T lymphocyte. (NIAID) 29 Sept. 2023. A company with a process to generate new thymus tissue for immune system functions is receiving a $37 million award from the government’s health breakthrough agency. The Advanced Research Project Agency – Health or ARPA-H is awarding the funds to Thymmune Therapeutics in Cambridge, Massachusetts for its work on engineering thymus tissue from stem cells.
Thymmune Therapeutics is a four year-old biotechnology company, spun off from the lab of genomics researcher George Church in the Wyss Institute at Harvard University. The company says its process recreates engineered thymus tissue for people without a functioning thymus. The thymus is a gland in the body’s lymphatic system, a network of tissue and organs that make up part of the immune system, that trains T lymphocytes, key white blood cells for fighting off infections. T lymphocytes or T-cells are initially generated from bone marrow, where they travel to the thymus for maturation. The thymus also produces hormones in the body’s endocrine system that support the immune system.
The thymus is most active in childhood and generally shrinks as part of the aging process, making older people more susceptible to diseases from a failing immune system. The company cites data showing some 10,000 people a year are diagnosed with thymus-related disorders, either from birth defects, cancer treatments, or autoimmune diseases.
Thymus cells and tissue in larger quantities
The Thymmune technology is based on research done originally at University of California in San Francisco led by Audrey Parent, now with UC-San Francisco’s Diabetes Center, that generated thymic tissue from human embryonic stem cells. The company says its process has advanced to where induced pluripotent stem cells, iPSC or so-called adult stem cells, can now transform into functioning thymus cells. And, says the company, they can produce engineered thymus cells and tissue in larger quantities, aided by machine learning algorithms.
ARPA-H is an agency in the U.S. Department of Health and Human Services with a mission to generate breakthrough technologies to advance human health, particularly where conventional research and development processes cannot do the job. In March, ARPA-H issued its first open broad agency announcement for proposals for R&D across a range of patient populations and diseases. This week, ARPA-H announced its first seven awards from the call, either contracts or cooperative agreements, of which Thymmune Therapeutics was the only industry recipient.
The ARPA-H award calls for Thymmune Therapeutics to first refine its lab process for transforming adult stem cells into thymus cells and tissue to produce T-cells. In the second part of the project, the company expects to demonstrate transplantation and engraftment techniques for engineered thymus to achieve immune functions in lab animals. If all phases of the project are achieved, the company can gain the full $37 million award.
“Drawing from decades of dedicated research on the thymus gland,” says Thymmune Therapeutics founder and CEO Stan Wang in a company statement released through BusinessWire, “our approach has the potential to revolutionize immunology through the creation of innovative therapies for patients in need with a range of immune system disorders.” Wang adds, “This funding will empower us to reshape drug development by harnessing cutting-edge advancements in thymus biology, iPSC technology, and machine learning.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 28th, 2023  (romnyyepez, Pixabay. https://pixabay.com/photos/cardiac-pulse-time-series-statistics-4240338/) 28 Sept. 2023. A new company developing digital cardiac diagnostics is collaborating with the Mayo Clinic on early detection of coronary artery disease, a key trigger of heart attacks. AccuLine in Petah-Tikva, Israel says the licensing agreement also calls for Mayo Clinic in Rochester, Minnesota to make an investment in the company, but further financial details are not disclosed.
AccuLine, founded in 2022, is developing a system called CORA to quickly and non-invasively collect key indicators of a person’s heart and breathing activity, such as cardiac electrical signals, blood oxygen saturation, and respiratory rhythm. The company says a single four-minute CORA test by any clinician yields data for analysis with artificial intelligence algorithms that creates a 3-D image of the heart’s electrical activity and helps calculate two key electrical signals. Those two indicators, says AccuLine, offer an assessment of an individual’s coronary arteries and likelihood of coronary artery disease.
Most current diagnostics for coronary artery disease or CAD, says the company, are either less accurate, require invasive procedures, or expensive. AccuLine says a clinical study shows the CORA system returns true-positive sensitivity of 85 percent, with comparable true-negative specificity.
One of Acculine’s founders and its chief medical officer is Aaron Frimmerman, director of the interventional cardiology unit at Hillel Yaffe Medical Center in Hadera, Israel. Frimmerman studies coronary artery disease, a condition caused by plaque accumulations in arteries, and the most common form of heart disease in the U.S. His research includes studies of imaging and other diagnostics for detecting the disorder.
“Proactively identify individuals at high risk”
“We are confident,” says Frimmerman in an AccuLine statement released through Cision, “that the advancement of the technology will pave the way for a solution to address a pressing unmet need related to the world’s leading cause of mortality.” He adds, “we are not only striving to transform CAD detection but also aiming to empower health care professionals with a tool that can proactively identify individuals at high risk for heart attacks.”
The agreement, says AccuLine, calls for the company and Mayo Clinic to collaborate on advancing CORA for the U.S. market, with the goal of making the system the preferred diagnostic tool for detecting coronary artery disease. AccuLine and Mayo Clinic will conduct a comprehensive clinical study needed for review by the Food and Drug Administration, and tailor the system’s workflow for adoption by primary care providers and cardiologists.
AccuLine says the agreement gives Mayo Clinic a know-how license, in effect rights to non-patentable elements in the technology, and Mayo Clinic is taking an equity stake in the company. No other details of the deal are disclosed.
Moshe Barel, CEO of AccuLine notes, “Mayo Clinic’s extensive clinical experience and expertise in diagnosing and treating cardiac conditions are important assets as we refine CORA, enabling the identification of individuals at high risk for heart attacks years in advance.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 27th, 2023  Southern bentwing bat (Steve Bourne, Wikimedia Commons) 27 Sept. 2023. Initial results from a clinical trial show an experimental vaccine produces immune responses in healthy adults against Covid-19 variants and a range of other coronaviruses. VBI Vaccines Inc. in Cambridge, Massachusetts released findings today from the trial on its web site, which are not yet peer-reviewed.
VBI Vaccines is a biotechnology enterprise developing vaccines invoking the immune system to treat and prevent disease, including cancer and hepatitis-B as well as infectious diseases. The company’s technology is based on virus-like particles, components of viruses designed to generate immune responses against pathogen targets, but not enough to cause the disease itself. In their natural state, says VBI, virus-like particles invoke immune responses against only a few viruses.
Thus, the company’s technology designs synthetic particles against viruses to resemble viral components, with a protein core between lipid or natural oil layers, which VBI calls enveloped virus-like particles or eVLPs. These eVLPs, says VBI, can produce a wider range of immune responses without infecting recipients.
VBI is developing several vaccines protecting against Covid-19 and coronaviruses in general. As reported by Science & Enterprise in Mar. 2021, VBI Vaccines received a $33 million award from Coalition for Epidemic Preparedness Innovations or CEPI for the company’s eVLP vaccine against Covid-19 Beta variants. At the time, the company was also working on a vaccine to protect against a broader range of known coronaviruses including the earlier SARS and Middle East respiratory syndrome or MERS, as well as Covid-19.
Protect against coronaviruses from bats and pangolins
The clinical trial assessed the company’s candidate vaccine code-named VBI-2901 against the original Covid-19 strain and SARS-CoV-2 variants that emerged since 2019 — Beta, Delta, and Omicron — as well as original SARS and MERS coronaviruses. The company says the vaccine is also designed to protect against coronaviruses from bats and pangolins, animals with viruses that can also infect humans. VBI says it collaborated with the National Research Council of Canada, CEPI, and the Canadian government’s Strategic Innovation Fund on the project.
The early-stage trial enrolled 101 healthy adult volunteers in Canada, who already received two or three messenger RNA vaccines against Covid-19 at least six months before the study. Participants were randomly assigned to receive two injections of VBI-2901 at low or high doses, 5 or 10 micrograms, or one dose at 10 micrograms. The study team tested blood samples from participants for antibody concentrations before and after vaccination to measure their immune responses. In addition, the study team followed up with participants after one and up to six months for blood tests.
VBI Vaccines says all participants show high or sustaining neutralizing antibodies against all of the Covid-19 variants and animal coronaviruses within 28 days following injections of VBI-2901, with peak immune responses shown by individuals receiving the single 10 microgram dose. The greatest increases in neutralizing antibodies, says the company, occur in people with the lowest antibody concentrations starting out.
In addition, single-dose recipients continue to exhibit neutralizing antibody titers or concentrations of about 75 percent from the initial tests against original the Covid-19 strain and other variants after five months following vaccination. VBI released limited safety data, noting only that reactions to VBI-2901 are similar to other eVLP vaccines, with no serious or life-threatening adverse effects reported.
Jeff Baxter, VBI Vaccines CEO says in a company statement the findings demonstrate “an ability to safely broaden durable, protective levels of immune responses and significantly boost neutralizing responses in participants with low baseline antibody titers.” The company says it received funds from CEPI and the Canadian government for further clinical trials.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 26th, 2023  SatioRx device (Satio Inc.) 26 Sept. 2023. A developer of medical devices designed as skin patches received a contract to develop a device to deliver any drug approved by FDA for delivery through the skin. Advanced Research Projects Agency for Health or ARPA-H awarded Satio Inc. in Boston $3.5 million to develop the remotely activated and disposable drug-delivery patch device.
The SatioRx device, says the company, seeks to make vaccine and drug delivery through the skin easier and less painful for individuals. The device, says Satio, uses a single design to deliver a wide array of liquid drugs or vaccines in standard vials with microscale needles that penetrate only the outer skin layers without causing pain, but still reach blood vessels to deliver the payloads. The company says it makes SatioRx for people in their homes, where the device can be activated to deliver a precise drug dose through telemedicine without having to visit a clinic.
Satio says its patch device can be particularly helpful for vaccine delivery. Delivery through the skin, says the company, enables lower doses of vaccines, reducing the risk of many adverse effects. Home administration of vaccines would reduce the need for clinic visits and make it easier for people with fear of conventional syringe needles, as well as speed delivery of vaccines to remote areas to better combat public health emergencies.
Small Business Innovation Research award
ARPA-H is the U.S. government’s advanced research agency for encouraging development of high-impact health technologies that meet pressing public needs not easily addressed through conventional methods. The agency, part of the U.S. Department of Health and Human Services or HHS, is modeled after the Defense Advanced Research Projects Agency, with projects that recruit multiple performers to address specific health challenges.
For Satio, the ARPA-H project is a Small Business Innovation Research or SBIR contract, where the agency sets-aside part of its research funds for U.S.-based small businesses. In this case, the award calls for development of a disposable drug delivery device, worn on the skin, with microscale needles for delivery into the blood stream. The patch device would enable delivery of any drug or vaccine approved by FDA for liquid delivery though the skin. As specified by ARPA-H, the device would also be activated by remote signals through telemedicine.
“At-home drug and vaccine delivery,” says Satio founder and executive chair Namal Nawana in a company statement, “offer transformational potential for better outcomes, efficiency, and lowering of overall costs of health care. The SatioRx novel design incorporates proprietary technologies to remotely deliver a broad range of drugs and vaccines transdermally.” Nawana is also founder of Sapphiros, an investor in diagnostics and other medical device companies, including Satio.
Satio is developing as well a family of wearable blood collection systems from simple dried blood dots to blood sample collection and combination blood draws and diagnostics. In Aug. 2023, Satio receive a contract from the Biomedical Advanced Research and Development Authority or BARDA, also part of HHS, to adapt the company’s integrated blood draw and diagnostic device to detect Ebola virus infections.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 23rd, 2023 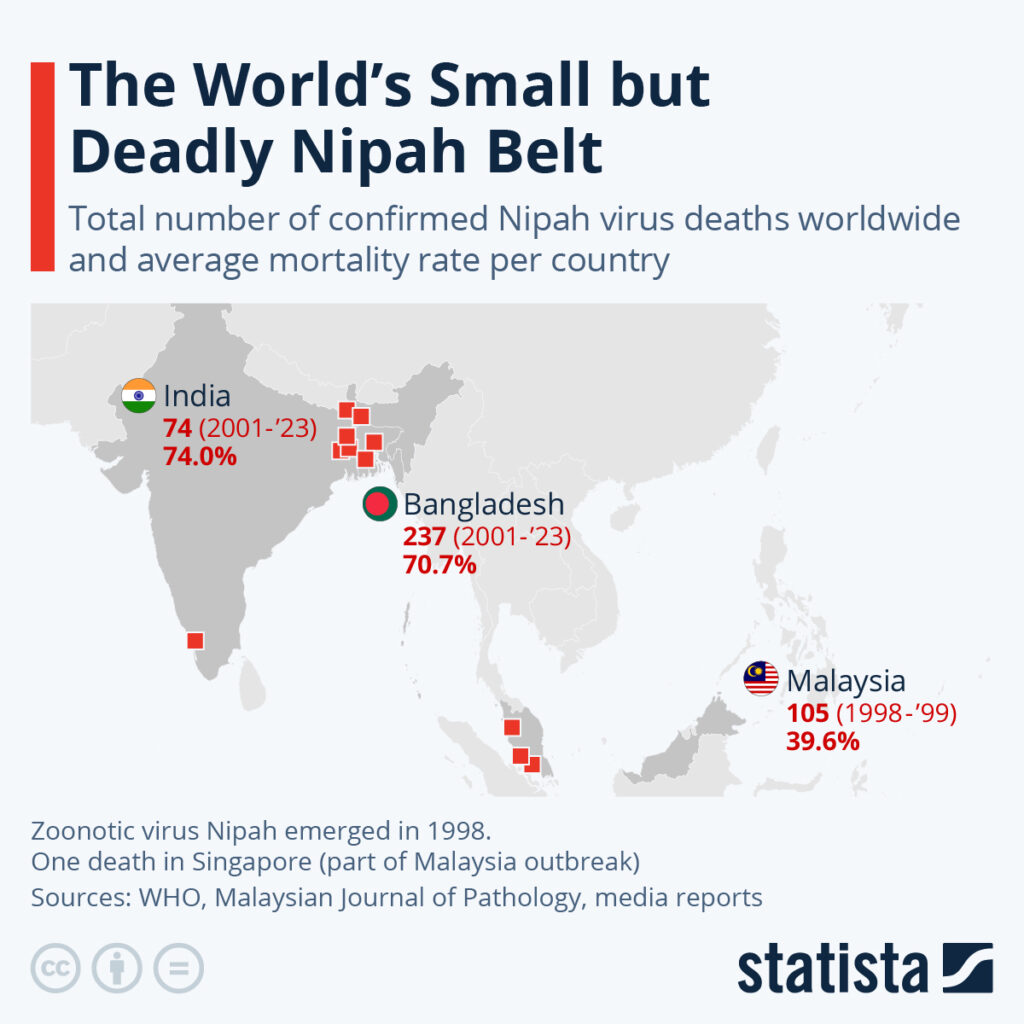 Click on image for full-size view. (Statista) 23 Sept. 2023. Nipah virus is a rare but deadly infectious disease affecting south and southeast Asia, and since August the Kerala state in India recorded six Nipah cases leading to two deaths. The business research company Statista this week published a map showing known Nipah virus cases in India, Bangladesh, and Malaysia since 1998.
Nipah is a zoonotic disease, one that transmits from animals to humans. In Kerala, the source is believed to be fruit bats, but bats can also affect domestic and farm animals, such as pigs, that can pass the virus on to humans. According to the journal Nature, Nipah virus is spread among humans mainly through contact with bodily fluids, but infections can cause fever, vomiting, respiratory problems and brain inflammation, with deaths occurring in 40 to 75 percent of cases.
Nature reports authorities in Kerala closed some schools, offices, and public transportation and tested some 700 people so far for the disease. As reported in Science & Enterprise in Jan. 2021, Moderna Inc. is developing a messenger RNA vaccine for Nipah virus, with three early-stage clinical trials either completed or underway testing Nipah vaccines.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 22nd, 2023  3-D print of influenza virus (National Institute of Allergy and Infectious Diseases, NIH) 22 Sept. 2023. A start-up biotechnology company says it received the okay for its investigational new drug application of an anti-viral drug to treat a whole class of seasonal flu strains. Via Nova Therapeutics Inc. says the Food and Drug Administration clearance allows the company to proceed with clinical trials of its lead product code-named VNT-101.
Via Nova Therapeutics is a two year-old enterprise in Oakland, California that develops drugs to treat acute or temporary viral diseases, such as seasonal influenza and rhinovirus or the common cold, that affect large numbers in the population and for vulnerable groups can have serious consequences. The company is advancing anti-viral drugs licensed from drug maker Novartis addressing acute infections, as opposed to chronic viral diseases such as HIV or hepatitis. While most of Via Nova’s pipeline is designed for respiratory conditions, one of its candidates targets the BK virus that poses a problem for kidney transplant patients.
VNT-101 is an anti-viral treatment for influenza-A, one of the two major types of influenza with influenza-B responsible for seasonal flu. Influenza-A is also responsible for flu pandemics, often with new strains emerging and mutations that affect large numbers in a population without immunity. Within the influenza-A sub-type are 130 known combinations so far of hemagglutinin and 11 neuraminidase proteins — H1 to H18 and N1 to N11 — that public health authorities must deal with each year. In addition, many of these strains are zoonotic, meaning they jump from birds and other animals to humans.
Key role in viral replication
Via Nova says VNT-101 targets the influenza-A nucleoprotein, one of a common set of proteins that interacts with other components in the virus during the infection process. The nucleoprotein also plays a key role in viral replication, and since it is common to all influenza-A strains, blocking this part of the virus is believed to help limit its infectiousness, as well as overcome resistance in further mutations.
An investigational new drug application or IND is a request to FDA to begin testing of a new drug or biologic therapy on humans. Applicants are asked to furnish preclinical research findings, usually lab and animal tests, with details on manufacturing the proposed treatments, data from related human clinical trials, and background information about the lead investigator of the trial. In some cases, FDA may ask for consultations with the requesting company before issuing an IND.
“We look forward to evaluating VNT-101 in the clinic,” says Via Nova co-founder Don Ganem in a company statement released through Cision. “This is an important step to developing a flu antiviral with a novel mechanism of action, and a milestone for Via Nova Therapeutics as we advance our first compound into the clinic.”
Ganem founded Via Nova Therapeutics with Kelly Wong in 2021 to advance four antiviral candidates they helped develop and subsequently licensed from Novartis. In Sept. 2021, Via Nova raised $20 million in its first venture funding round from biotechnology investor Aditum Bio.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 21st, 2023  (Gordon Johnson, Pixabay) 21 Sept. 2023. A developer of treatments with psychoactive compounds for brain disorders is partnering with a lab at Yale University to assess methylone as a PTSD therapy. Transcend Therapeutics in New York and the labs of two psychiatry professors at Yale University medical school are evaluating the drug methylone to treat post-traumatic stress disorder, as part of a $1 million project funded by the U.S. Department of Defense.
PTSD is a chronic psychiatric condition with symptoms such as recurring flashbacks, dreams, and fearful episodes, often triggered by a person’s own traumatic or dangerous experiences. While many people may experience flashback episodes in the days after a fearful experience, continuing symptoms long after an event are signs of PTSD. Combat veterans, victims of abuse, and disaster survivors are PTSD candidates. The National Center for PTSD, part of the U.S. Department of Veterans Affairs, estimates six in 100 U.S. adults will experience PTSD at some point.
Transcend Therapeutics is a three year-old biotechnology enterprise seeking therapies from psychotropic or mind-altering substances for neuro-psychiatric disorders including PTSD. The company says its goal is to offer compounds exhibiting psychoactive effects that can help people with PTSD, depression, and related disorders by working faster and with shorter durations, but without causing hallucinations. Science & Enterprise reported on Transcend Therapeutics’ emergence from stealth mode in Feb. 2023, where the company also raised $40 million in its first venture round.
The company’s lead product is a form of methylone, in a class of psychoactive agents known as entactogens that include MDMA, better known on the street as ecstasy. With methylone, however, Transcend Therapeutics believes people with PTSD can experience similar therapeutic effects without the unwanted adverse effects nor long ramp-up times of MDMA. The company is testing its methylone therapy in an early- and mid-stage clinical trial in the U.K. among participants with PTSD.
Identify neuro-biological mechanisms and dynamics
While the trial is underway, Transcend Therapeutics is also documenting methylone’s mechanism of action to treat PTSD. In June, Yale University received a $1 million award from Department of Defense to study the ways MDMA and methylone work in preclinical studies. The three-year project plans to identify the neuro-biological mechanisms and dynamics in the brain affected by entactogens to better understand their therapeutic affects.
Transcend is working with Yale psychiatry professors Alfred Kaye and Christopher Pittenger on the project that focuses on ways these compounds affect the plasticity of neurons in the brain. Kaye studies the reprogramming of neurological circuits in response to trauma with images of neurotransmitter sensors, electrophysiology, and computational biology. Pittenger is co-founder of Yale’s psychedelic science program that seeks to better understand how mind-altering compounds like LSD and psilocybin affect brain functions and behavior.
“We know from a published clinical case series and other research,” says Transcend Therapeutics co-founder and CEO Blake Mandell in a company statement, “that methylone could hold tremendous promise for the treatment of PTSD. Yale’s research, funded by the Department of Defense, will provide invaluable data on the neuroplastic effects of methylone on the brain.”
Benjamin Kelmendi, a Transcend Therapeutics co-founder and Yale University colleague of the researchers adds, “There is a tremendous demand, especially among veterans, for new and effective treatments for PTSD.” Kelmendi serves as an advisor to the company.
More from Science & Enterprise:
Updated: 28 Sept. 2023
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 20th, 2023  (Gerd Altmann, Pixabay. https://pixabay.com/illustrations/dna-analysis-research-7237234/) 20 Sept. 2023. A genomics company is providing precision medicine analytics from its databases with generative A.I. based on large language models from Amazon Web Services. Dante Genomics in New York says its collaboration with AWS will make clinical advice available to users, but also identify cases where a physician’s involvement is needed.
Dante Genomics is a seven year-old company that provides whole genome sequencing and analysis for individuals. As the name implies, whole genome sequencing queries the entire human genome, including regions not normally associated with disease or coding for proteins. Genes that produce instructions for proteins, called the exome, make up a small portion of the entire genome, and is the region most often sequenced and analyzed. As computer processing and data storage costs decline, however, sequencing the entire genome becomes more feasible, particularly when seeking answers for complex or rare conditions. Dante says whole genome sequencing results in 20 times more data than exome sequencing.
The company offers a series of standard reports from its analytics for common disease types such as cancer and heart disease, as well as rare diseases. Dante Genomics says it also produces customized reports that consider factors such as medical history, family history, and ethnic background. And the company says its reports can highlight latent carrier conditions and potential adverse effects to drugs not yet encountered. Dante also offers its software as a service called Clinical Lab in a Box for internal use by hospitals or clinics, as well as software offered over the web for one-time analysis of genomic data provided by the user.
Choose a model that best fits data sets
The collaboration with Amazon Web services aims to provide generative artificial intelligence from Dante’s whole genome data sets arrayed on the AWS Bedrock platform. The Bedrock platform, says AWS, provides large language models as a service through an application program interface or API. Large language models are machine learning algorithms trained by very large data sets such as text or image collections, and providing prospective outcomes, made famous recently by ChatGPT. In the Bedrock service, AWS says client companies can choose from a selection of foundation models that best conform to their data sets for machine learning, and test the pipelines — workflow processes for model and data generation — without investing in their own machine learning or A.I. infrastructure.
Dante Genomics says it plans to provide generative A.I. from the Bedrock platform, with customers interacting with the system through text chats. The company says it expects the system to produce genetic inferences to calculate the likelihood of disorders such as heart disease, respiratory conditions, or seizures. In addition, says Dante Genomics, the system will identify results where a physician should be consulted.
“By partnering with the tech experts at AWS,” says Dante Genomics co-founder and CEO Andrea Riposati in a company statement released through Cision, “Amazon Bedrock as a platform will help us make great strides in revolutionizing the everyday application of genomic data in personalized medicine, delivering better health outcomes with genomic data as a foundation.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 19th, 2023  Land mine detection team, in Tajikistan, 2014. (U.N. Development Programme, Flickr. https://flic.kr/p/mCRMKB) 19 Sept. 2023. Researchers from a university biochemistry lab and company developing synthetic enzymes designed techniques using engineered bacteria to detect buried land mines at longer distances. A team from Enzymit in Ness-Ziona, Israel and Hebrew University of Jerusalem describe their process in the 22 Aug. 2023 issue of the journal Computational and Structural Biotechnology Journal.
Land mines with buried unexploded artillery and bombs are a continuing threat to the lives of people in former war zones, even decades after the end of hostilities. Land mines are placed underground, designed to explode by the presence or proximity of humans, whether combatants or civilians. Similar buried weapons are designed when triggered to project shrapnel fragments through the air causing multiple deep wounds across a wider area. According to the latest Landmine Monitor report published in Nov. 2022 by the International Campaign to Ban Landmines, these weapons continue to kill and maim civilians, as well as disrupt lives and essential services in more than 60 countries, with more than 5,500 killed or injured in 2021, mostly civilians.
Moreover, detecting and clearing land mines today is dangerous and laborious work. Highly trained workers are needed to carefully explore suspected mine fields, often with handheld detection devices. Because combatants do not always document land mine placement, large areas often need to be examined, making it a slow and inefficient process.
Chemicals indicating the presence of explosives
The Environmental Microbiology and Biosensor Laboratory at Hebrew University led by microbiologist Shimshon Belkin studies biological sensors that harness living cells, modified genetically to express proteins that sense and report the presence of target substances. Among the lab’s work is detection of harmful or toxic biological and chemical molecules, but also sensing of specific chemicals indicating the presence of explosives, such as 2,4,6-trinitrotoluene and 2,4-dinitrotoluene, better known as TNT and DNT respectively.
Enzymit is a three year-old enterprise founded by computational biologists Gideon Lapidoth and Dror Baran — now CEO and chief operating officer respectively — who studied protein design at Weizmann Institute of Science, a research university in Israel. The company offers a process for designing synthetic enzymes with specified chemical and biological properties mainly for industrial and commercial use, from deep learning algorithms. Additional algorithms, says Enzymit, adapt and refine the chemistry to enable high-speed assembly of enzymes for subsequent testing and production. Data produced from this development process, adds Enzymit, are used to further train the company’s algorithms. The company says its cell-free design process is simpler and less expensive than conventional synthetic biology techniques based on fermentation.
The research team led by Belkin adapted work by Enzymit to extend its biological sensing process based on E. coli bacteria to detect trace amounts of DNT, an indicator of land mines and unexploded ordinance. Based on genetic screening and computational analytics by Enzymit, the researchers identified alterations of a gene in the bacterium’s DNA to enable expression of a protein with greater sensitivity to DNT molecules by the microbe, and to generate more powerful bioluminescent signals when sensing this chemical. In lab assessments, including tests using beach sand, the altered E. coli sensors exhibit a detection threshold seven times lower than before, with 45 times the signal intensity, and 40 percent faster response time.
Enzymit says the findings can be the basis for more sensitive devices to detect chemicals in soil indicating land mines from a longer distance than current technologies, making the process safer for workers. “This project marks a breakthrough in the field of land mine detection,” says Lapidoth in a company statement released through Cision, “while demonstrating the incredible potential of harnessing the synergy between synthetic biology and A.I. for a future where humanitarian and environmental challenges can be met with safe and sustainable solutions.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|











 RSS - Posts
RSS - Posts
You must be logged in to post a comment.