Subscribe for email alerts
Donate to Science & Enterprise
|
By Alan, on September 18th, 2023  (Sergio Santos, nursingschoolsnearme.com, Flickr) 18 Sept. 2023. A crowdsourced competition offered by NIH is seeking new technologies to monitor the health and detect early problems with new babies and their mothers, at the point of care or in the home. The Fetal Monitoring Challenge, conducted under the The Rapid Acceleration of Diagnostics Technology or RADx Tech program at National Institutes of Health, has a total prize purse of $2 million and an initial proposal deadline of 17 Nov. 2023.
According to Centers for Disease Control and Prevention, maternal death rates in the U.S. have risen each year from 17.4 per 100,000 births in 2018 to 32.9 in 2021. Among non-Hispanic Black communities, the rate is more than double, with 69.9 maternal deaths per 100,000 births in 2021. In addition, NIH cites data showing some 2 million stillbirths occur each year worldwide, with 21,000 stillbirths occurring in the U.S., and 40 percent of those deaths taking place after the onset of labor. Nonetheless, says NIH, fetal monitoring is conducted unevenly in the U.S. and elsewhere in the world, with few new technologies in development.
The Fetal Monitoring Challenge aims to generate new technologies making it simpler and easier to monitor fetal and maternal health, including in the home, during late pregnancy and delivery. The need is particularly acute in low-resource communities, often with limited access to high-quality prenatal care. Thus, the challenge is looking for devices that provide actionable information at the point of care or at home, and have reasonable prospects for market entry within five years in low-resource areas in the U.S. or worldwide.
Solutions are expected to directly detect or monitor health indicators of a fetus, or abnormalities in the umbilical cord or placenta, as well as be non- or minimally-invasive. Examples of technologies to be considered are wearable devices, diagnostic systems using smartphones or tablets, integrated sensing or imaging, and digital health platforms.
At least working prototypes
Registration opens today with details provided on the Challenge.Gov web site. Participants can enter the competition as members of independent teams or representatives of established institutions, organizations, or businesses. A webinar for entrant prospects is scheduled for 5 Oct., and initial proposals are due 17 Nov. 2023. Proposals are expected to describe technologies that are at least in working prototype stage, with data supporting the device’s operations. Ideas for technologies still in conceptual or design stages will not be accepted. The Point of Care Technology Research Network is taking registrations and entries, and managing the challenge for NIH.
The challenge’s initial proposals will be judged during November and December 2023, with the first round of up to 10 semi-finalists announced in mid-December. The second phase of the competition takes place in January and February 2024, with up to six finalists expected by March 2024. From the finalists, the first three place winners and runners-up will be announced by October 2024. Each of the semi-finalists receives $5,000 while each finalist gains $75,000. First, second, and third place winners receive $750,000, $400,000, and $200,000 respectively, and runners-up each win $50,000.
“By bringing attention to an unacceptable state of care,” says Bruce Tromberg, director of the National Institute of Biomedical Imaging and Bioengineering or NIBIB, in an institute statement, “we hope to inspire innovators to engineer solutions for accurate, cost-effective, and safe fetal monitoring and diagnostic approaches. The goal is safer birth outcomes, focusing on the health of the fetus in the latter stages of pregnancy, when easy-to-use technologies might detect the need for specific medical care.” NIBIB is sponsoring the challenge with National Institute of Child Health and Human Development, also part of NIH, and the Bill & Melinda Gates Foundation.
NIH began the RADx Tech program in April 2020 to jump-start development of new point-of-care and home-based tests for Covid-19 infections. Science & Enterprise reported on the start of the initiative, and several products and services spawned by the program, most recently in July 2023 — funding of a combined one-time test for flu, Covid-19, and respiratory syncytial virus or RSV infections done at home.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 17th, 2023 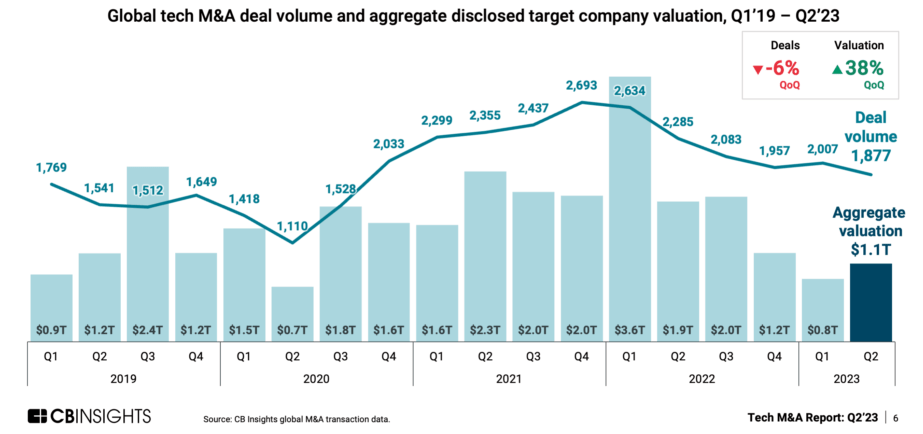 Click on image for full-size view (Statista) 17 Sept. 2023. The value of mergers and acquisitions in the global technology sector rose for the first time in over a year in the second quarter of 2023, while M&A deal counts dropped somewhat. The data, reported earlier this month by technology industry intelligence company CB Insights (registration required), show merger and acquisition valuation and deal numbers in the tech industry worldwide have returned to about the same levels as before the Covid-19 pandemic.
CB Insights reports tech industry M&As worldwide totaled $1.1 trillion from April through June 2023, an increase of 38 percent from the first quarter total of $800 billion. As the chart shows, the second quarter gain in M&A valuation is the first sizable increase since the first quarter of 2022, when the sector recorded a monster $3.6 trillion in deals. At the same time, the number of global tech industry M&A deals in Q2 2023 fell by six percent to 1,877 from 2,007 in Q1. Nonetheless, these quarterly valuation and deal totals are roughly the same as reported in 2019, before the onset of Covid-19.
The reports also indicates tech industry merger hunters are favoring larger acquisition targets. CB Insights says the number of tech M&A targets valued at $1 billion or more rose by 21 in the second quarter, while the median valuation of those targets nearly doubled to $45 million, the first gain in three quarters. And 41 percent of tech industry M&A targets valued at more than $100 million in Q2 were in the U.S. In addition, the median number of employees at tech companies acquired in Q2 rose to 359, rising sharply from 214 in Q1, and after falling for three straight quarters.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 15th, 2023  (Seanbatty, Pixabay) 15 Sept. 2023. I.T. systems company Fujitsu Ltd. is providing artificial intelligence model generation and fairness assessment software as open-source projects hosted by the Linux Foundation. The Linux Foundation in San Francisco says it approved incubating the two initiatives in late August, and announced the collaboration ahead of the organization’s Open Source Summit Europe conference that begins next week in Bilbao, Spain.
Fujitsu, based in Tokyo, offers software from its A.I. platform code-named Fujitsu Kozuchi, unveiled in April 2023. The company says Fujitsu Kozuchi is designed for collaborative and interactive development with customers that generates core models such as machine learning and generative A.I., with additional business components applied as needed. The added business components offer features such as defect inspection and analysis, fraud monitoring, and consumer analytics. Fujitsu says it holds 970 patents related to A.I.
Find an optimum solution and predictive model
Linux Foundation and Fujitsu seek to encourage further rapid development of A.I., but acknowledge the need to make A.I. tools more accessible to users without advanced I.T. skills, as well as ensuring all user populations are accurately reflected in algorithms. The SapientML project, one of the joint initiatives, generates machine learning models and code from data presented in tabular form. Data sets in tables train the models to produce a workflow for predicting outcomes in a new data collection. SapientML works in successive stages to identify and test machine learning components, winnowing them down to construct a process for finding the optimum solution and a predictive model. The software was presented in May 2022 at the International Conference on Software Engineering.
Intersectional Fairness, the other Fujitsu project, seeks to detect and mitigate biases against population groups in A.I. algorithms that may be hidden in model training data. The process, as described in an earlier paper, assesses the interaction of population attributes in outcomes such as gender or age with comparative statistical tests and real-world data sets to identify biases in training data. The model further evaluates the combination of those attributes and adjusts for biases without impairing accuracy of the underlying model.
Both the SapientML and Intersectional Fairness projects are available as open-source software or OSS on GitHub. Fujitsu Kozuchi includes automated machine learning model generation and ethics-for-fairness among its core modules. “Offering A.I. technologies as OSS to developers worldwide,” says Fujitu’s chief technology officer Vivek Mahajan in a Linux Foundation statement, “opens up new opportunities for innovation across various industries by lowering the barrier of entry.” Mahajan is scheduled to give one of the keynote speeches at the Open Source Summit Europe conference next week.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 14th, 2023  (National Heart, Lung, and Blood Institute, NIH) 14 Sept. 2023. A new company spun-off from a university lab consortium is advancing gene therapies delivered through the lungs to treat rare inherited respiratory diseases. The work of AlveoGene in Oxford, U.K. is based on discoveries from the U.K. Respiratory Gene Therapy Consortium or GTC, a group of researchers studying inherited respiratory diseases at universities of Oxford and Edinburgh, and Imperial College London.
A technology developed by GTC researchers uses engineered lentiviruses to deliver gene therapies. Among the well-known lentiviruses is HIV, but other lentivirus types offer the ability to deliver genes to cells for long durations. Studies by GTC researchers have enabled the modifying of lentiviruses for safe and efficient gene delivery into cells of epithelium tissue lining the airways. The consortium researchers engineered lentiviruses further for better airway cell delivery, with key proteins from Sendai viruses that affect mice. And the labs are adapting gene therapies for inhaled delivery with hand-held nebulizer devices that create a fine aerosol mist.
The GTC’s first major disease target was cystic fibrosis, a progressive inherited respiratory disease from mutations in the cystic fibrosis transmembrane conductance regulator or CFTR gene. In Oct. 2021, the consortium licensed its technology to drug maker Boehringer Ingelheim, giving Boehringer exclusive worldwide rights to develop and commercialize GTC’s lentiviral delivery technology for gene therapies treating cystic fibrosis. Part of that deal involved manufacturing processes for lentiviral gene therapies from Oxford Biomedica, a company featured in a March 2019 Science & Enterprise report.
Symptoms similar to emphysema
AlveoGene, formed last year, is gaining an exclusive license for GTC’s advanced lentivirus-delivery technology for disease targets other than the CFTR gene associated with cystic fibrosis. The licensed technology includes the ability to deliver gene therapies with an aerosol nebulizer. AlveoGene’s lead product, code-named AVG-001, is an inhaled treatment for alpha-1 antitrypsin deficiency or AATD, a rare inherited lung disease. People with AATD have symptoms similar to emphysema, but can also lead to scar tissue or cirrhosis in the liver. Earlier this year, the Food and Drug Administration cleared an at-home test for mutations responsible for AATD, which is expected to increase the number of diagnosed cases. AlveoGene says AVG-001 is currently in preclinical development.
The company is raising seed funds from Oxford Science Enterprises or OSE that creates and finances start-up businesses based on research at its university, and Old College Capital or OCC, the venture investment arm of Edinburgh Innovations supporting new companies from that university’s labs. Joining the seed round is Harrington Discovery Institute, affiliated with University Hospitals in Cleveland, Ohio, but also a funder of life science start-ups in the U.K. Investment amounts were not disclosed.
“The combination of pioneering science, an extensively validated platform, access to world-leading expertise through our founding scientists and the backing of OSE, Harrington, and OCC, provides a fantastic foundation for the company,” says AlveoGene executive chair David Hipkiss in a company statement released through Globe NewsWire. “This will enable AlveoGene to rapidly advance our first candidate – AVG-001 a unique, inhaled gene therapy for AATD – towards clinical development.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 13th, 2023  Healthy neuron illustration (NIH, Flickr. https://flic.kr/p/CW1m6P) 13 Sept. 2023. A new award from National Institutes of Health funds preclinical work to prepare for a clinical trial assessing a treatment to reverse brain signaling damage in people with Alzheimer’s disease. The $3 million grant from National Institute on Aging, an agency of NIH, funds the two-year program advancing an experimental drug made by Spinogenix Inc. in San Diego.
Spinogenix, founded in 2016, discovers compounds for restoring functions of synapses, the electro-chemical signaling components of neurons in the brain, damaged by neurodegenerative diseases. The company says its lead product code-named SPG302 is an oral drug derived from benzothiazole, a common pharmaceutical and industrial chemical. In an earlier preclinical study, researchers from Spinogenix and university labs found SPG302 increases the density and protein expression of synapses, as well as cognitive functions in lab mice induced with neurodegenerative conditions. That team also found the changes in synapses and functions occur without affecting amyloid-beta or tau proteins on neurons associated with these disorders.
The company is testing SPG302 in an early-stage clinical trial in Australia, looking mainly for safety among healthy volunteers, but also among patients with amyotrophic lateral sclerosis or ALS. The research team is evaluating adverse effects among small numbers of healthy volunteers and ALS patients to determine safe single and multiple daily doses of SPG302, but also chemical effects in the body including concentrations of the drug in blood plasma, and interactions with food. In addition, the Australian team is measuring effects on brain activity among healthy participants, as well as respiratory and functional outcomes among ALS patients.
Reverse declines in cognitive and motor function
The National Institute on Aging award supports further preclinical research on SPG302 as a therapy to restore functioning synapses in people with Alzheimer’s disease, to prepare for an investigational new drug application with the Food and Drug Administration, in effect a request to begin clinical trials. The grant funds safety and toxicology testing of SPG302 for four weeks in lab rats and dogs, and manufacturing of SPG302 as a daily oral drug with a process that meets pharmaceutical industry standards, known as good manufacturing practice or GMP.
“Our approach is unique for diseases of the central nervous system,” says Spinogenix founder and CEO Stella Sarraf in a company statement. “SPG302 is an innovative treatment focused on regenerating synapses to reverse declines in cognitive and motor function, which is different than the many other therapeutics that are being developed for neurodegenerative conditions, which mostly aim to slow the degenerative process.”
The award is a Small Business Innovation Research or SBIR grant made under NIH’s small business programs that set aside a part of the agency’s research funding for U.S.-based and owned companies. Most SBIR grants are made in two parts: a first phase to determine technical and commercial feasibility, and a second phase to develop and test a working prototype or prepare for clinical trials. This is a second-phase project, with the earlier preclinical studies of SPG302 funded in part 1.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 12th, 2023  (Gordon Johnson, Pixabay. https://pixabay.com/vectors/mental-health-cranium-head-human-3350779/) 12 Sept. 2023. A new company developing treatments for neurological and psychiatric diseases from aberrant immune system activity is raising $58 million in seed funds. Arialys Therapeutics in La Jolla, California is a one year-old enterprise founded by former researchers at Astellas Pharma Inc. in Tokyo, and advancing a therapy acquired from that company.
Arialys Therapeutics addresses autoimmune disorders in the central nervous system, where the immune system mistakes healthy cells and tissue for invading pathogens. The company’s lead product, code-named ART5803, is designed to block autoimmune antibodies causing a form of encephalitis or swelling of the brain. The disorder, called anti-NMDA receptor encephalitis, is caused by aberrant antibodies attacking N-methyl-D-aspartate or NMDA receptor proteins in the synapses or signaling components of neurons in the brain. While anti-NMDA receptor encephalitis is the most common form of autoimmune encephalitis, it’s still a rare disease, and according to Arialys, poorly managed and often misdiagnosed.
Reverses mental and motor abnormalities
ART5803, says Arialys, is designed to block the autoimmune antibodies responsible for anti-NMDA receptor encephalitis or ANRE. The treatment is a synthetic protein resembling an immunoglobulin G antibody that binds to a specific epitope or binding site in the aberrant autoimmune antibody to block its activity in the central nervous system or CNS. In a paper for the American Academy of Neurology annual meeting in April 2023, Arialys Therapeutics co-founder and chief scientist Mitsuyuki Matsumoto reported on preclinical research showing in lab cultures ART5803 binds to autoimmune antibodies from ANRE patients. In further tests with marmoset primates induced with ANRE, infusions of ART5803 reverse mental and motor abnormalities caused by the autoimmune antibodies within two weeks.
Arialys says the Food and Drug Administration assigned orphan drug status to ART5803, which the company calls a precision medicine for treating neurological and psychiatric disorders. “Precision medicine approaches have led to tremendous successes in oncology,” says Matsumoto in a Arialys Therapeutics statement, “and now we have the insights to be at the forefront of developing precision medicines to transform the treatment landscape for neuropsychiatry.” Matsumoto adds that the company plans “to file an IND next year and advance ART5803 into the clinic for the treatment of ANRE and autoimmune psychosis.” An investigational new drug application or IND is, in effect, a request to FDA to begin clinical trials.
Arialys Therapeutics is raising $58 million in seed funds from life science investors Avalon BioVentures, Catalys Pacific, MPM BioImpact, Johnson & Johnson Innovation, and Alexandria Venture Investments, with the proceeds aimed at expanding its portfolio to other CNS autoimmune diseases. The company is currently located at the Avalon BioVentures accelerator in La Jolla, with Jay Lichter, managing partner at Avalon BioVentures, serving as president and CEO of Arialys.”With the ability to isolate and characterize pathogenic autoantibodies in the CNS,” notes Lichter, “we can now develop novel therapeutics and diagnostics to help millions of people who suffer from neuropsychiatric disorders driven by autoimmune disease.”
More from Science & Enterprise:
Disclosure: The author owns shares in Johnson & Johnson.
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 11th, 2023  (National Cancer Institute) 11 Sept. 2023. Results of a clinical trial show one radiologist using software with artificial intelligence can detect more breast cancer cases in mammograms than current reviews by two radiologists. Findings from the trial conducted by researchers at Karolinska Institutet in Stockholm, Sweden using a cancer diagnostics system by Lunit Inc. in Seoul, South Korea, appear in Friday’s issue of the journal The Lancet Digital Health.
Mammography remains a basic tool for breast cancer screening, which requires interpretation of the breast images by trained radiologists. In Europe and other regions, current medical practice calls for two radiologists to review the images. However, the authors cite their own earlier study showing a wide variation in interpretations by 110 radiologists of mammogram images compared to a subsequent diagnosis of breast cancer. In addition, say the authors, trained radiologists for interpreting mammograms are in short supply. Thus the researchers evaluated a system using A.I. algorithms to reduce the need for two radiologists to interpret mammogram images.
The study called ScreenTrustCAD used Lunit’s Insight MMG system that the company says employs deep learning algorithms constantly updated with new results to inspect mammogram images for suspicious lesions indicating cancer. Lunit says the system improves cancer detection even in dense or fatty breast tissue that can make mammograms more difficult to analyze. The company says its Insight systems have received FDA clearance in the U.S. and CE marks in Europe. In addition, the authors earlier assessed three A.I. commercial diagnostics technologies and rated the Insight MMG system as the top performer for use in this study.
Fewer false positives and reduced workloads
The researchers evaluated human and A.I. image interpretation across a wide population in Sweden to find if algorithm-assisted detection is at least equivalent to a diagnosis made by radiologists. The study team tested mammograms taken at a hospital in Stockholm from 58,344 women age 40 to 74 in 2021-22, with images from 55,581 included for analysis. All mammogram images were first reviewed by radiologists, then assigned for review either by another radiologist or the Lunit system. The researchers looked primarily for diagnosis of breast cancer within three months of the mammogram, with detection rates for a radiologist plus A.I. system, compared to review by two radiologists. The team also compared assessments by a single A.I. system and evaluations made by two radiologists plus the A.I. system.
Results show assessments by two radiologists detected breast cancer in 250 women, while one radiologist and the A.I. system found 261 cancer cases or four percent more than the conventional two-radiologist method. Using the A.I. system alone detected cancer in 246 women, within the statistical confidence interval of two radiologists, while adding A.I. to the two radiologists detects 269 cancer cases. The findings also show fewer false positive readings by the combination of radiologist and A.I. system compared to two radiologist, and reduced workloads on radiologists.
The authors indicate the human and machine assessments complement each other in detecting cancer in mammograms. “A.I. and humans perceive images slightly differently,” says radiologist and first author Karin Dembrower in a Karolinska Institutet statement, “which creates a synergy that improves our chances of detecting cancer.” Senior author Magnus Strand, a cancer pathologist at Karolinska Institutet adds, “It’s clear to us that for screening mammography, one A.I.-supported radiologist is a better alternative than two radiologists without A.I.”
“A.I. is redefining cancer screening standards,” notes Brandon Suh, CEO of Lunit in a company statement, adding “This study signifies a milestone in health care, ushering in an era where A.I. seamlessly complements and elevates the standards of breast cancer screening.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 9th, 2023 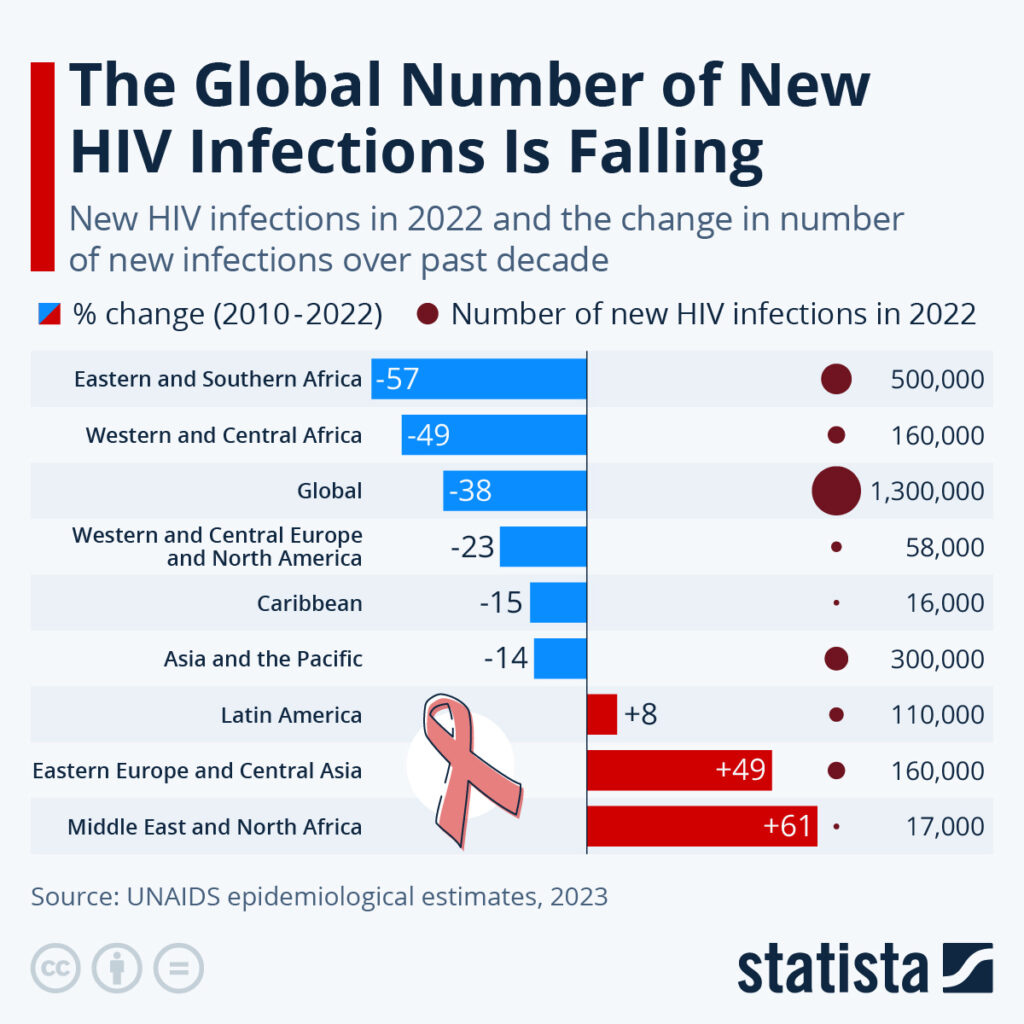 Click on image for full-size view. (Statista, https://www.statista.com/chart/30760/new-hiv-infections-in-2022-and-the-change-in-number/) 9 Sept. 2023. The number of new infections from the human immunodeficiency virus or HIV declined worldwide last year and over the past decade, with the sharpest declines in sub-Saharan Africa. A report from the United Nations agency UNAIDS, issued in July, provides the data compiled this week in chart form by the business research company Statista.
HIV attacks lymphocytes or white blood cells in the immune system, thus weakening the body’s natural defenses against other infectious diseases, including tuberculosis. Infections from HIV can progress to acquired immunodeficiency syndrome or AIDS, a deadly condition. People with HIV can spread the virus through exchanges of bodily fluids, such as semen or blood, through sex, sharing syringes by drug users, or from mother to child in the womb.
Worldwide, an estimated 1.3 million new HIV cases were reported in 2022, of which about 660,000 or half occurred in eastern, southern, central, or western Africa. Yet, as the chart shows, those regions recorded the sharpest declines in new infections since 2010, from 49 percent in western and central Africa to 57 percent in eastern and southern Africa. Worldwide, HIV infections fell by 38 percent since 2010, according to UNAIDS. At the same time, however, HIV cases rose by 61 percent since 2010 in the Middle East and northern Africa, while infections in eastern Europe and Central Asia gained nearly half (49%). Together those regions account for an estimated 177,000 cases in 2022.
Some of the declines in HIV infections can be traced to the U.S. President’s Emergency Plan for AIDS Relief, or PEPFAR, a program started under the George W. Bush administration that provides HIV medications such as antiretroviral drugs to not-for-profit organizations worldwide, as well as support for local health care systems to prevent HIV and AIDS. As reported today by Associated Press, however, some Republican members of Congress threaten to withhold further funds from PEPFAR due to accusations of support for abortions by grantees.
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 8th, 2023  (Patrick Mannion, Flickr. https://flic.kr/p/9UZtg) 8 Sept. 2023. Researchers use stem cells to generate human neurons resembling those in the brain that control breathing during opioid reactions, for eventual chip devices to test overdose therapies. A team from University of Central Florida and the organ chip-model developer Hesperos Inc. describe their findings in yesterday’s issue of the journal Advanced Biology.
According to Centers for Disease Control and Prevention, the U.S. is now in a third wave of opioid overdose deaths, driven almost entirely by synthetic opioids such as fentanyl. CDC says the number of drug overdose deaths jumped more than 16 percent from 2020 to 2021, the last full year of data, with three-quarters of the 107,000 overdose deaths from opioids. When overdoses occur, breathing quickly becomes slower and shallower, a condition called opioid-induced respiratory depression and a major cause of overdose death.
Researchers led by James Hickman, professor of chemistry at University of Central Florida, are seeking more reliable methods than current animal models to test new treatments for opioid overdoses. The key brain region for regulating automatic respiratory rhythm, say the authors, is the preBötzinger Complex, also associated with heart rate and blood pressure. But up to now, preclinical tests of new treatments for opioid overdoses such as naloxone use lab rodents, which have a preBötzinger Complex region in their brains, but does not function the some way as humans. Thus, say the authors, a preclinical testing process is needed with human preBötzinger Complex neurons to provide better assessments of safety and efficacy.
Linked to simulate multiple organ functions
Hickman is also co-founder and chief scientist at Hesperos Inc. in Orlando, Florida that develops models of human organs on small chip devices for testing new drugs for toxicity, safety, and efficacy. The company’s technology recreates functions of organs, with fine channels and wells engraved into clear plastic, lined with human cells and tissue, and connected electronically for measurement and control. Hesperos says its organ models can also be linked together to simulate multiple organs. The company says its devices include brain models.
In the paper, Hickman and colleagues including Hesperos staff created a model for testing opioid overdose treatments with neurons like those found in the human preBötzinger Complex. The authors say they produced the neurons by transforming induced pluripotent stem cells, so-called adult stem cells, which they confirmed by testing for characteristic biomarker proteins and electrophysiological responses. The researchers then tested the generated neurons with escalating doses of four natural and synthetic opioid compounds: codeine, methadone, fentanyl, and the opioid peptide DAMGO. The results show characteristic reactions to the four opioids by inhibiting the firing of the generated neurons. A subsequent test also shows characteristic reactions to the presence of naloxone by recovering activity in the neurons earlier exposed to opioids.
Generating neurons similar to those in the human preBötzinger Complex, say the authors, is the first step in creating a chip device emulating opioid overdose. Hesperos says the next stages will integrate a brain chip with the generated neurons into a multi-organ model that includes liver, heart, kidney, and skeletal muscle to simulate reactions to opioid overdoses in other parts of the body. Hickman says in a Hesperos statement that the researchers’ work “will provide invaluable insights into understanding opioid addiction and recovery, paving the way for more effective treatments and ultimately saving lives.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
By Alan, on September 7th, 2023  (B_Me, Pixabay. https://pixabay.com/photos/pedestrians-people-busy-movement-400811/) 7 Sept. 2023. A new crowdsourced competition seeks more robust early indicators of Alzheimer’s disease and related dementias using analytics powered by artificial intelligence. The challenge with a total purse of $650,000 is sponsored by National Institute on Aging or NIA, part of National Institutes of Health, and hosted by the data science crowdsourcing company DrivenData, with an infrastructure provided by challenge technology company HeroX.
NIA says early detection of Alzheimer’s disease and related dementias is hampered by a lack of sensitive tools to identify these disorders in their earliest stages, when more treatment options are available. The agency says it created the challenge to find solutions for all populations affected by dementias like Alzheimer’s disease, particularly those individuals often left behind by current detection techniques. Challenge participants are expected to specifically address health disparities among older segments of the population spelled out in NIA’s health disparities research framework that identifies environmental, sociocultural, behavioral, and biological factors related to research on aging.
The competition has three parts, beginning with identification of more inclusive and shareable data sets on variables contributing to cognitive decline and aging that offer a more representative foundation than available today for building algorithms to detect Alzheimer’s and related diseases. Participants are expected to identify a wide range of sources, including research findings funded by NIH or others, real-world data such as electronic medical records and insurance claims, social media posts, and data from personal devices.
First submissions in Jan. 2024
The competition’s first stage expects to award a total of $200,000 that includes bonuses of $25,000 for proposals addressing populations disproportionately impacted by Alzheimer’s and related dementias, and data sets addressing biases in current sources. Summary drafts for this part of the challenge are due on 17 January 2024, with full submissions due on 31 January. Judging of entries is done in two stages, first for finalists and then winners, with the winners announced in Sept. 2024.
The second part of the challenge asks participants to write algorithms and analytics using the data sources generated in part 1, judged on accuracy, openness, addressing biases, and ability to explain their mechanisms, scheduled to begin in Sept. 2024. In the competition’s third stage, participants are asked to put together their work on data source identification and algorithm building into a single package for early detection of Alzheimer’s disease and related dementias, with top teams presenting their solutions at an “innovation event.” The third part of the challenge is scheduled for Mar. to Sept. 2025.
While NIA is sponsoring the competition, the challenge is offered through DrivenData, a social enterprise organization staging competitions with outcomes affecting public policies and civil society, based on artificial intelligence and data science. DrivenData, in Denver, Colorado, hosts the competitions and provides a process for evaluating machine learning and other algorithms and analytics submitted by participants. The challenge technology is provided by HeroX in Vancouver, British Columbia and several sites in the U.S. HeroX offers its technology as a turnkey service with templates for competition design, crowdsourcing promotion, evaluation, awards, and project management.
“Being able to detect dementia diseases early can transform outcomes for impacted patients,” says Kal Sahota, CEO of HeroX in a statement released through Cision. “This challenge,” adds DrivenData co-founder Greg Lipstein, “is a powerful opportunity to leverage advances in data and A.I. to equip care teams with greater capabilities for early prediction.”
More from Science & Enterprise:
We designed Science & Enterprise for busy readers including investors, researchers, entrepreneurs, and students. Except for a narrow cookies and privacy strip for first-time visitors, we have no pop-ups blocking the entire page, nor distracting animated GIF graphics. If you want to subscribe for daily email alerts, you can do that here, or find the link in the upper left-hand corner of the desktop page. The site is free, with no paywall. But, of course, donations are gratefully accepted.
* * *
|
Welcome to Science & Enterprise Science and Enterprise is an online news service begun in 2010, created for researchers and business people interested in taking scientific knowledge to the marketplace.
On the site’s posts published six days a week, you find research discoveries destined to become new products and services, as well as news about finance, intellectual property, regulations, and employment.
|











 RSS - Posts
RSS - Posts
You must be logged in to post a comment.