St. Jude Medical Inc., a medical device developer in St. Paul, Minnesota, received a CE mark indicating regulatory approval in Europe for its Eon line of neurostimulators to treat chronic migraine. The CE mark, an acronym for the French Conformité Européene, indicates a product meets safety, health and environmental protection requirements in the 27 EU member states, as well as Iceland, Liechtenstein, and Norway.
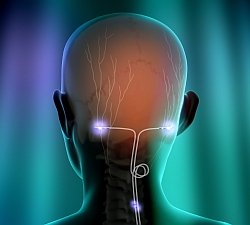
The Eon neurostimulators deliver peripheral nerve stimulation of the occipital nerves to manage the pain and disability associated with chronic migraine headache. The occipital nerves are located just beneath the skin in the back of the head. A small electrical lead or leads are placed under the skin and connected to the neurostimulator, which produces the mild electrical pulses of stimulation.
The St. Jude neurostimulators, both the Eon and earlier Genesis line, are designed for patients suffering pain for at least four hours a day and 15 days a month, as well as encountering at least moderate disability. Neurostimulator patients also should also have not responded to three or more prevention drugs. The company says the Eon device is designed for longer battery life and can be configured for more complex pain management scenarios.
Last year, St. Jude received CE approval for its Genesis line of neurostimulators, which also delivers peripheral nerve stimulation to the occipital nerves. A double-blind, randomized clinical trial of the Genesis devices reported last year that patients who received stimulation reported a 28 percent decrease in their number of headache days — seven fewer days a month — compared to the placebo group that reported a 4 percent decrease. The neurostimulator patients also reported significantly lower scores on questionnaire disability indexes.
A migraine is a headache that may occur with symptoms such as nausea, vomiting, or sensitivity to light. Migraine.com reports more than 37 million people in the U.S. suffer from migraines, with 2 to 3 million of those cases considered chronic.
Read more:
- Chronic Pain Technology Company Gains $3.5M Series B Funds
- Patent Awarded for Migraine Drug Inhalation Method
- CE Mark Approved for Migraine Implant Therapy
- Study Explains How Tinted Lenses Offer Relief from Migraines
* * *

 RSS - Posts
RSS - Posts
[…] European Approval Granted for Implanted Migraine Device […]