14 January 2015. U.S. Food and Drug Administration today approved a device blocking nerve signals between the brain and stomach to help obese people who have not responded to other weight-loss methods control their appetites and lose weight. The Maestro Rechargeable system approved by FDA is made by medical device maker EnteroMedics Inc. in St. Paul, Minnesota.
EnteroMedics develops systems that treat obesity and metabolic disorders by blocking vagus nerve signaling between the brain and stomach. The vagus nerve extends from the brain to the abdomen, through the esophagus, lungs, and heart, and is part of the involuntary nervous system controlling various bodily functions including digestion.
The company’s technology, called Vbloc, controls signaling along the vagus nerve, targeting perceptions of hunger and fullness that respond to expansion of the stomach and contractions of stomach muscles. Blocking these signals is believed to help individuals better control their appetite and food intake, thus encouraging weight loss. These same signals are also believed to affect secretion of digestive enzymes and blood glucose levels.
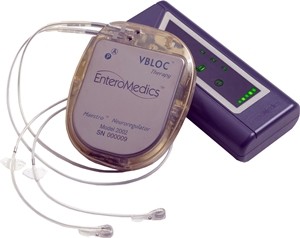
The Maestro rechargeable system incorporates Vbloc technology in a device about the size of a heart pacemaker implanted in the abdominal region. Although electric stimulation is known to block nerve activity between the brain and the stomach, the specific mechanisms for weight loss due to the device are still unknown.
FDA approved the device for patients aged 18 and older who fail to lose weight with a weight-loss program, and who have a body mass index of 35 to 45 with at least one other obesity-related condition, such as type 2 diabetes. The approval is based in part on a clinical trial of 233 patients with a body-mass index of 35 or higher, who received either an activated Maestro device or a de-activated device for comparison.
The trial shows after 12 months, patients with the full Maestro device lost 8.5 percent more excess weight than patients with the de-activated device. But the trial did not meet its objective of patients with the full Maestro device losing 10 percent or more of their excess weight, compared to those with the deactivated device. Nonethless, about half of those in the trial with the Maestro device (53%) lost at least 20 percent or more of their excess weight, with 38 percent losing 25 percent.
In the trial, patients reported adverse events including nausea, pain at the neuroregulator site, vomiting, as well as surgical complications. Other adverse events included pain, heartburn, problems swallowing, belching, mild nausea and chest pain.
FDA says its advisory panel on weight loss devices found the evidence collected over 18 months supported approval of the Maestro device, and agreed the device’s benefits eclipsed their risks. FDA also cited a separate survey of candidates for weight-control devices who say they would accept risks of surgically-implanted devices given the amount of weight loss achieved.
As part of the approval, FDA asked EnteroMedics to conduct a follow-up study of 100 or more patients to track their weight-loss progress and collect further safety, adverse reaction, and efficacy data.
Read more:
- Trial Shows Weight Loss Drug Can Help Stop Smoking
- Pfizer, Second Genome to Partner on Human Microbes, Obesity
- Pfizer Licenses University Institute’s Obesity Research
- Trial Shows Smartphone App Effective for Weight Loss
- Asthma Drug Reveals Potential as Diabetes, Obesity Treatment
* * *

 RSS - Posts
RSS - Posts
[…] FDA Approves Nerve Pathway Device for Obesity […]