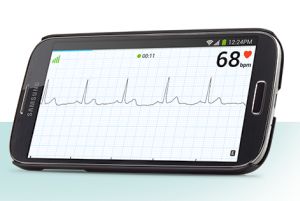
22 January 2015. An algorithm analyzing signals to detect atrial fibrillation from a heart monitor built into mobile devices received regulatory approval in Europe. AliveCor, a developer of heart monitoring systems for mobile devices, says the company received the Conformité Européene or CE mark for the algorithm that analyzes heart monitoring signals in its AliveECG App.
The CE Mark signifies approval to market regulated products such as medical devices in the European Union and associated countries, but the company says the AliveECG app is now available only in the U.K. and Ireland. The AliveECG app analyzes signals from AliveCor’s heart monitor app to detect atrial fibrillation. If the heart rhythm pattern suggests atrial fibrillation, the AliveECG app notifies the user. The data, stored online, can also be shared with physicians.
The AliveCor heart monitor works with Apple/iOS and Android mobile devices, and is intended for use by physicians for patients with known or suspected heart conditions, as well as health-conscious patients. In August 2014, the Food and Drug Administration approved the AliveECG algorithm for use in AliveCor systems sold in the U.S.
Atrial fibrillation, or AFib, is an irregular heartbeat that can lead to stroke or heart failure, and affects some 2 million people in the U.K. and Ireland, as well as 2.7 million Americans. With AFib, heart muscle contractions in the upper chambers beat irregularly instead of in a regular rhythm, which can cause blood to pool and lead to blood clots, including those that move to the brain and cause a stroke. Some 15 to 20 percent of stroke victims have this kind of irregular heartbeat, and left untreated, people with AFib are 4 to 5 times more likely to suffer a stroke.
While some people with AFib report symptoms, such as a racing heartbeat or light-headedness, many people with the condition experience no symptoms, making it difficult to detect. AliveCor, in San Francisco, says the AliveECG app reports the condition in real time for quick action by patients and physicians, which can be particularly important for people who do not experience symptoms.
Beginning in June 2014, AliveCor established heart monitoring centers at pharmacies in the U.K. and Ireland , where store customers could test for atrial fibrillation with the company’s heart monitor app. Community screenings of this kind are important, says the company, since about one-third of cases with AFib have not yet been diagnosed.
Read more:
- FDA Issues Draft Wellness Device Guidance
- Behavioral App Developer Lands $20 Million in Venture Funds
- Flexible Pulse Oximeter Designed for Wearable Devices
- IBM, Health Tech, Univ Designing Critical Care Mobile System
- Smartphone App Screens Infants for Jaundice
* * *

 RSS - Posts
RSS - Posts
[…] Mobile Heart Monitor Algorithm Approved in Europe […]