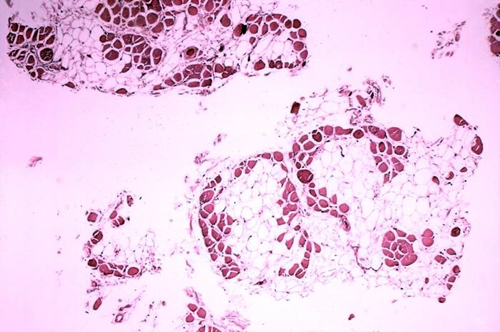
Cross-section of muscle tissue from a person with Duchenne muscular dystrophy shows extensive replacement of dark colored muscle fiber with light-colored adipose or fat cells. (Centers for Disease Control and Prevention)
24 July 2015. Biotechnology company Fibrogen Inc. says the U.S. Food and Drug Administration approved an application to test its fibrosis drug candidate in patients with Duchenne muscular dystrophy. The company says FDA’s approval was part of a new drug application for its candidate, code-named FG-3019, already in intermediate-stage clinical trials as a therapy for various fibrotic diseases.
Duchenne muscular dystrophy is a rare genetic disorder resulting in muscle degeneration and weakness, primarily in the shoulders, arms, hips, and thighs. The disease affects mainly boys starting at age 3 to 5, and caused by a defective gene that fails to produce the protein dystrophin for strengthening muscle fiber and protecting muscles from injury.
FG-3019 is an engineered antibody designed to block the activity of connective tissue growth factor or CTGF, a protein secreted by cells that provides structure and support for those cells. While CTGF plays an important role in skeletal development and wound repair, it is also associated with fibrosis, the growth of excess tissue that occurs in response to injury or damage, and is often referred to as scarring. The excess tissue growth, however, can happen internally as well with damaging effects on organs in disorders such as liver fibrosis and idiopathic pulmonary fibrosis, as well as some forms of cancer.
In Duchenne muscular dystrophy, says the company, the lack of dystrophin leads to diminished muscle function that correlates with the extent of intra-muscular fibrosis. Fibrogen says it studied the role of CTGF in Duchenne muscular dystrophy for 10 years, and believes CTGF prevents the repair of damaged muscles in people with the disorder. In preclinical studies, says Fibrogen, FG-3019 reduced muscle fibrosis and improved muscle function.
FG-3019 is currently in intermediate stage trials for idiopathic pulmonary fibrosis that affects the lungs and liver fibrosis that results from chronic hepatitis B infections. The company says FG-3019 reversed fibrosis in a sizable number of patients enrolled in the idiopathic pulmonary fibrosis trial. The company is also enrolling participants for a trial to test FG-3019 among patients with pancreatic cancer.
Fibrogen, in San Francisco, says it shared its preclinical findings and met with the TREAT-NMD Advisory Committee for Therapeutics, a group that reviews and provides guidance on drug development research for neuromuscular diseases. The meetings with this committee, says the company, led to refinements in its clinical trial design. Fibrogen adds the advisory committee and other experts support plans for the clinical trial planned for enrollment later this year.
Read more:
- Trial Testing Respiratory Muscle Drug for ALS Patients
- Foundation Supporting ALS Pilot Clinical Trials
- Patient Registry Started for Rare Genetic Disorder
- Rare Disease Therapy Company Raises $60 Million in IPO
- Trial Begins Testing Sickle Cell Blood Therapy
* * *

 RSS - Posts
RSS - Posts
[…] FDA Clears Trial of Fibrosis Drug for Muscular Dystrophy […]