19 August 2015. A clinical trial shows an implanted system did a better job of lowering blood glucose levels in people with type 2 diabetes, than an approved glucose control drug. Intarcia Therapeutics in Boston, developer of the system code-named ITCA 650, released top-line results of the late-stage trial testing the device against the drug Januvia made by Merck.
ITCA 650, consists of exenatide, a drug that acts like a hormone in the body known as incretin to help control blood sugar. Exenatide, like incretin, prompts the pancreas to release insulin, and at the same time prevents the hormone glucagon from stimulating the liver to release sugar into the blood stream. In addition, the drug helps slow the rate at which the stomach empties after a meal, keeping an individual feeling full longer, thus also keeping blood sugar from rising too quickly after eating.
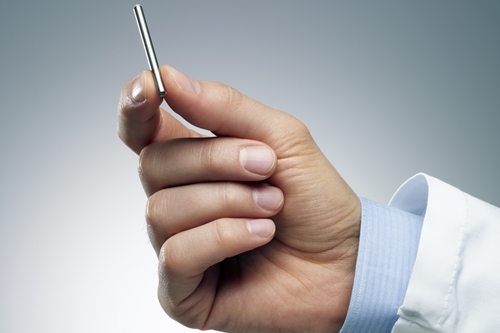
The use of exenatide and other drugs acting like incretin is limited by the need for people with type 2 diabetes to self-inject the drug twice a day, with nausea a common side effect. ITCA 650 also includes a delivery method for exentatide, where a pump the size of a matchstick is inserted under the skin to provide a continuous dose of the drug through osmosis for up to one year without replacement. Because the pump is inserted under the skin, notes Intarcia, a qualified clinician can quickly remove it if needed.
The clinical trial tested ITCA 650 against the glucose control drug sitagliptin, marketed as Januvia by Merck. Sitagliptin, taken once a day, acts by increasing natural substances in the body for controlling blood glucose. The drug is usually prescribed as a therapy along with changes in diet and exercise.
Some 535 individuals with type 2 diabetes were recruited for the study, randomly assigned to receive the ITCA implant dispensing 60 micrograms of exenatide per day, or the drug sitagliptin in doses of 100 milligrams per day. The research team looked primarily at blood glucose levels, defined as concentrations of glucose in hemoglobin, a protein in red blood cells, abbreviated HbA1c or A1c, over the course of one year. All participants had A1c levels between 7.5 percent and 10.5 percent, and were taking the drug metformin to decrease the glucose absorbed from food.
The results reported by Intarcia show after one year, more individuals in the trial with the ITCA 650 system achieved the recommended A1c target of 7 percent or less than those taking sitagliptin. In addition, more participants with the ITCA 650 system achieved an A1c reduction greater than 0.5 percent, combined with weight loss of 2 kilograms (4.4 pounds) or more, a secondary objective of the study. The company notes that ITCA 650 is not being tested as a treatment for obesity.
Intarcia released some details from the study about safety and tolerability of its treatments. Discontinuations for nausea were in low single digits, says the company, with minor events of hypoglycemia or low blood sugar in the low single digits among both the ITCA 650 and sitagliptin recipients. Insertions and removals of ITCA 650 implants, the company adds, were well tolerated with minor infections at the insertion site running less than 1 percent.
Intarcia says it plans to submit full details of the clinical trial for publication and presentations at professional meetings.
As reported in November 2014 in Science & Enterprise, Intarcia licensed ITCA 650 rights to the French pharmaceutical company Servier in a deal with a potential value of $1 billion. The agreement gives Servier exclusive rights to Intarcia’s diabetes treatment technology in regions outside of the U.S. and Japan.
Read more:
- Smart Insulin Patch Designed to Regulate Blood Glucose
- Trial Testing App to Manage Diabetes, Foot Ulcers
- Mobile Diabetes Device Maker Lands $20M in Venture Funds
- MedImmune, Joslin Partner on Diabetes Research
- Janssen, Foundation to Devise Diabetes Prevention Strategy
Hat tip: Fortune/Termsheet
* * *

 RSS - Posts
RSS - Posts
[…] and Google are joining a field of new devices to improve care and management of diabetes, with implanted systems and mobile apps from other companies already being tested in clinical […]