19 September 2016. The U.S. Food and Drug Administration is seeking a smartphone app that can quickly find a source of the antidote drug naloxone for someone having an opioid overdose. The competition has a top prize of $40,000, with registration available 23 September through 7 October 2016, on Challenge.gov, the U.S. government’s portal for crowd-sourcing challenges.
FDA, working with other federal agencies, wants to make it easier for people to find naloxone if they need it for someone having an opioid overdose. Opioids work by reducing the intensity of pain signals to the brain, particularly regions of the brain controlling emotion, which reduces effects of the pain stimulus. Examples of leading opioid prescription pain medications are hydrocodone, oxycodon, morphine, and codeine.
One of the collaborating agencies, Substance Abuse and Mental Health Services Administration or Samhsa, says some 3.8 million people in the U.S. age 12 or older in 2015 were current misusers of pain relievers. Centers for Disease Control and Prevention says 28,000 people in the U.S. died from an opioids in 2014, with 78 overdose deaths occurring every day. Since 1999, says CDC, the number of overdose deaths involving opioids, including heroin, more than tripled.
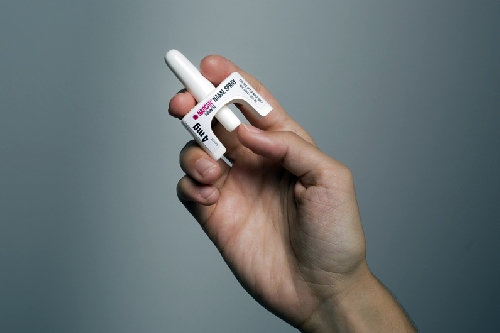
Naloxone is available by prescription in injectable form, including a self-injector device that can also be administered by care givers or family members. As reported by Science & Enterprise in November 2015, FDA approved a nasal spray formulation of naloxone, known as Narcan developed by Adapt Pharma.
While naloxone still requires a prescription, many states and communities are making the drug as readily available as possible, until an over-the-counter version of the drug is approved and on the market. The competition seeks to improve accessibility further by tapping into ideas from the information technology, entrepreneurial, medical, scientific, and public health communities for harnessing the power of mobile technology to connect laypersons including even bystanders to naxolone sources if they’re in the presence of someone experiencing an opioid overdose.
Peter Lurie, FDA’s associate commissioner for public health strategy and analysis, says in an agency statement, “Mobile phone applications have been developed to educate laypersons on how to recognize an overdose and administer naloxone, and to connect bystanders with individuals in need of other medical services, such as CPR. To date, however, no application is available to connect carriers of naloxone with nearby opioid overdose victims.”
FDA instituted a tight timetable for the competition. Participants can register for the challenge beginning on Friday, 23 September on the U.S. government’s crowd-sourcing portal, Challenge.gov, with registration open to 7 October. Registrants will be given access to information on the opioid epidemic, approved formulations of naloxone, recommendations for the safe and appropriate use of naloxone, and FDA guidance on mobile medical applications. The agency also plans a two-day coding event on the FDA White Oak campus in Sliver Spring, Maryland and virtually, on 19-20 October for participants to develop their apps further, including prototypes.
Challenge participants will then have to 7 November to refine their code and submit a video demonstrating their prototypes, as well as a summary description of the app. A panel from FDA, Samhsa, and National Institute on Drug Abuse at NIH will evaluate submissions, with the highest scoring entry receiving the top prize of $40,000. Participants may also apply for Small Business Innovation Research support from National Institute on Drug Abuse.
Read more:
- Challenge Seeks Health Technology Start-Ups
- Heart Research Built Into Personalized Activity Tracker
- Phone-Linked Glucose Meter Shows Health, Monetary Benefits
- Cancer Center Tests Mobile Patient Tracking
- App Evaluates Chest Pain Patients for Discharge
* * *

 RSS - Posts
RSS - Posts
[…] FDA Holding Opioid Overdose App Competition […]