4 May 2017. A 1 year-old enterprise developing gene therapies to treat rare inherited metabolic disorders is raising €37.5 million ($US 40.8 million) in its first venture funding round. This initial financing for Vivet Therapeutics, a biotechnology company based in Paris, was led by Novartis Venture Fund and Columbus Venture Partners.
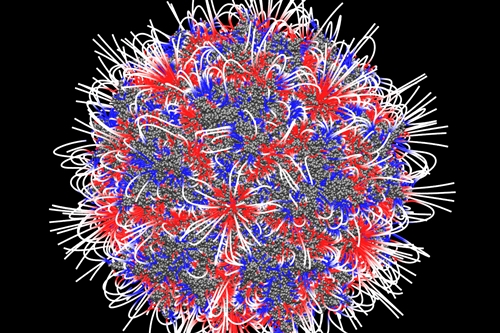
Vivet Therapeutics is developing gene therapies for rare inherited disorders of the liver delivered with adeno-associated viruses. In their natural form, adeno-associated viruses are benign and naturally occurring microbes that can infect cells, but do not integrate with the cell’s genome or cause disease in humans, other than at most mild reactions. The Vivet technology, however, uses synthetic forms of these viruses developed at and licensed from Centro de Investigación Medica Aplicada , or CIMA, at University of Navarra in Pamplona, Spain, and Massachusetts Eye and Ear in Boston.
The key synthetic adeno-associated virus adapted by Vivet is known as Anc80, developed at Mass. Eye and Ear. Labs at that institution designed Anc80 to overcome immune-system reactions to natural forms of these viruses, by recreating their evolutionary timeline and altering undesired proteins under the viral shell. Anc80 is derived from the most ancient of these evolving versions, which in tests with mice successfully addresses the retina, but also the liver.
Gloria Gonzalez-Aseguinolaza, director of the gene therapy program at CIMA, is a pioneer in research with gene therapies delivered by adeno-associated viruses, particularly for rare liver diseases. In 2016, she and 2 European biotech industry veterans founded Vivet Therapeutics, licensing the technology developed by her research at CIMA, as well as Anc80 from Mass. Eye and Ear for liver disorders. Gonzalez-Aseguinolaza continues as the company’s chief scientist.
Vivet’s lead program is a gene therapy to treat Wilson’s disease, an inherited disorder where the body cannot process excess copper. Wilson’s disease, says the company, affects 1 in 30,000 individuals, who require lifelong treatments to prevent build-ups of excess copper that cause liver and neurological damage. The disorder is traced to malfunctions in the ATP7B gene that produces a protein for processing copper in the body, allowing copper to accumulate in and damage the liver.
The company’s therapy for Wilson’s disease, code-named VTX801, is a truncated but healthy form of the ATP7B gene, delivered with synthetic adeno-associated viruses. Vivet says results of preclinical research with animals indicate VTX801 treatments are safe and effective. Funds from the initial financing are expected to advance VTX801, as well as gene therapies for other rare, inherited metabolic diseases.
Vivet’s first funding round is led by Novartis Venture Fund, an arm of the pharmaceutical company, that invests largely in early-stage life sciences companies producing innovative therapeutics, medical devices, diagnostics, and delivery systems. The round’s co-leader is Columbus Venture Partners that supports early-stage and high-growth life science enterprises in Spain, sometimes with participation by the Spanish government. Also taking part in the financing are Roche Venture Fund, HealthCap, Kurma Partners, and Ysios Capital.
More from Science & Enterprise:
- Trial to Test Gene Therapy for Inherited Eye Disease
- Messenger RNA Engineered to Produce Antibodies
- Duchenne Gene-Editing Company Launched, Raising $5M
- Patent Issued for Peptides in Diabetes Gene Therapy
- Synthetic Viruses in Gene Therapy Licensed for Production
* * *

 RSS - Posts
RSS - Posts
[…] that are delivered with synthetic adeno-associated viruses for rare, inherited metabolic diseases. €37.5 million in Series A funding. Based in Paris, […]