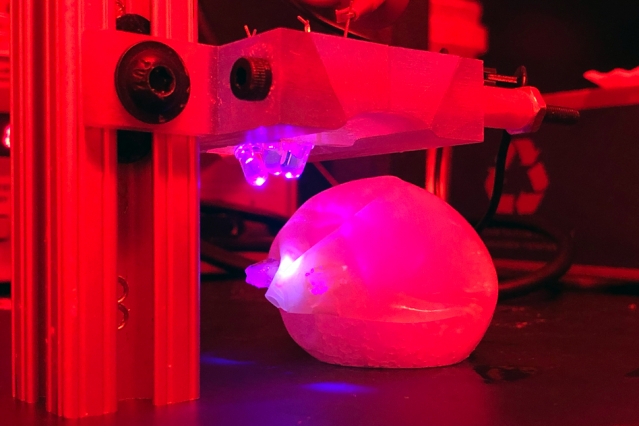
Bariatric balloon with a seal made of light-activated hydrogel, used in tests with pigs (Ritu Raman, MIT)
17 Jan. 2020. A medical engineering team developed a light-sensitive polymer gel that activates compounds to break down implanted or ingested devices. Researchers from Massachusetts Institute of Technology describe their material in today’s issue of the journal Science Advances.
The authors, from the biotechnology and engineering labs of Giovanni Traverso and Robert Langer at MIT, are seeking better methods for removing ingested or implanted medical devices, without resorting to invasive endoscopes. Traverso and Langer, as reported in Science & Enterprise, developed a number of ingested devices for diagnostics and long-term drug delivery, particularly in the gastrointestinal tract. And while tests show the devices can break down on their own when finished with their tasks, circumstances may require removing then before the end of their lifetimes.
“We are developing a set of systems that can reside in the gastrointestinal tract,” says Traverso in an MIT statement, “and as part of that, we’re looking to develop different ways in which we can trigger the disassembly of devices in the GI tract without the requirement for a major procedure.”
Up to now, their ingestible devices react to changes in changes in temperature, pH (acidity), or the presence of certain compounds to start breaking down. In this project, lead by postdoctoral researcher Ritu Raman in Langer’s lab, the researchers created a light-activated method to break down an ingested or implanted device on demand, rather than wait for certain conditions to emerge, and without invasive techniques.
The MIT team devised their solution with hydrogel, or water-based polymers, that can be formed into various devices. They started with polyacrylamide (PAAM) and poly2-acrylamido-2-methylpropane sulfonic acid (PAMPS), two bio-compatible, degradable — and importantly for this project, transparent — polymers used in implants such as artificial cartilage.
For these devices that operate inside the gastrointestinal system, the researchers needed materials both resilient and flexible, yet still break down when triggered. “You’re forming one polymer network and then forming another polymer network around it,” says Raman, “so it’s really entangled. That makes it very tough and stretchy.”
To provide the light-sensitive property, the researchers added in the compound ortho-nitrobenzyl, known to react to blue light for cleaving other bio-compatible polymers. Since the hydrogels would have to work inside the body, the system needed a simple light source, in this case a tiny, ingestible light-emitting diode, or LED. The researchers found they could alter the speed and extent of polymer degradation by adjusting amounts of ortho-nitrobenzyl, light wavelengths, and distance from the light source. Toxicity tests in the lab show no harmful effects from the materials on gastrointestinal cells.
The team created two applications to test their material, a seal on a bariatric balloon, a device implanted to treat obesity, and an esophageal stent used to open a blocked or narrowed esophagus, as occurs in esophageal cancer. The seal on the bariatric balloon opens a valve to deflate the balloon. In tests with pigs — animals with organs about the same size and functions as humans — the researchers found the light-activated hydrogel seal releases the valve, allowing the balloon to deflate. And the deflation speed can be adjusted, depending on the intensity of the light. The esophageal stent, also in tests with pigs, breaks down when activated by the LED and passes through the pigs’ gastrointestinal systems.
The team plans to test this approach with other implanted or ingested devices. “This study is a proof of concept that we can create this kind of material,” says Traverso, “and now we’re thinking about what are the best applications for it.” In 2015, Langer, Traverso, and entrepreneur Amy Schulman founded the company Lyndra Therpeutics Inc. in Watertown, Massachusetts to license and commercialize their extended release drug-delivery technology, where this light-activated material could be deployed.
More from Science & Enterprise:
- Once-a-Month Contraceptive Pill Being Developed
- Capsule Designed to Inject Insulin in Stomach Wall
- Bluetooth Data Added to Ingestible Capsules
- Patent Set for Long-Term Drug Capsules
- Precision Medicine Technique Devised for Brain Tumors
* * *

 RSS - Posts
RSS - Posts
You must be logged in to post a comment.