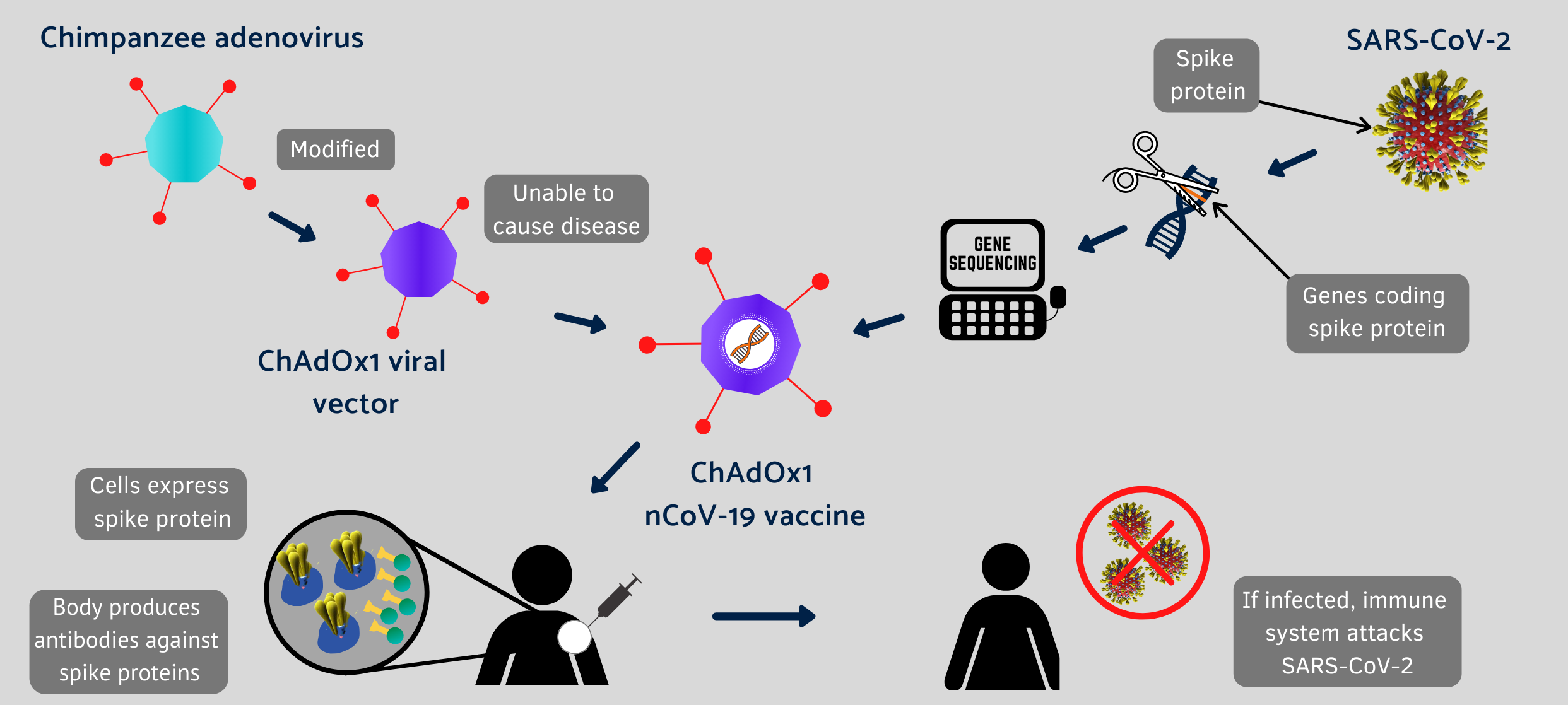
Oxford Covid-19 vaccine infographic. Click on image for full-size view. (Oxford Vaccine Group, Univ. of Oxford)
30 Apr. 2020. AstraZeneca agreed today to help develop, manufacture, and distribute a candidate vaccine to prevent Covid-19 infections designed by University of Oxford. The vaccine known as ChAdOx1 nCoV-19 results from research by the university’s Jenner Institute and Oxford Vaccine Group, and began testing in a clinical trial last week.
ChAdOx1 nCoV-19 is based on the ChAdOx1 vaccine framework, derived from an adenovirus in chimpanzees, similar to a virus in humans considered benign, or responsible at most for symptoms like the common cold. In this case, the adenovirus is genetically engineered to keep from replicating in humans, with genetic material added to make proteins similar to the characteristic spike glycoprotein on the surface of SARS-CoV-2 viruses. That spike protein binds to receptors in human cells, to gain entry and cause Covid-19 infections.
ChAdOx1 nCoV-19 is designed to generate an immune response to the spike protein that stops the protein from binding to receptors on human cells, thus preventing infections. The university says other vaccines made from the ChAdOx1 framework were tested so far in some 320 individuals, and found generally safe and well-tolerated, with adverse side effects reported including fever, headache, and soreness in the arm receiving the vaccine. The U.K. government is backing the program with £20 million ($US 25.2 million) in funding.
The deal with drug maker AstraZeneca comes less than a week after Oxford announced the start of a clinical trial testing ChAdOx1 nCoV-19. That trial is enrolling some 1,100 healthy adults in England randomly assigned to receive either ChAdOx1 nCoV-19 or the standard MenACWY vaccine approved to prevent against meningococcal infections that can lead to meningitis. The MenACWY vaccine is given for comparison instead of the usual saline solution to better gauge reactions to the Covid-19 vaccine, instead of adverse reactions to vaccines in general.
Under the new agreement, AstraZeneca in Cambridge, U.K. will work with the spin-off company Vaccitech to help further develop Oxford’s Covid-19 vaccine, as well as produce and distribute the vaccine, should initial trial results prove successful. Distribution will aim to get the vaccine in the hands of medical authorities in the U.K. as soon as possible, and internationally, including low- and middle-income regions. Final details, say the parties, are still being worked out.
All parties agree to cover the costs of development, manufacturing, and distribution, and not seek profits during the pandemic. Oxford and Vaccitech that licenses the technology will retain the rights to the vaccine, but the university says any royalties it receives following the pandemic will be invested in research, including a pandemic research center developed with AstraZeneca.
Sir John Bell, professor of medicine at Oxford, says in a statement, “We believe that together we will be in a strong position to start immunizing against coronavirus once we have an effective approved vaccine. Sadly, the risk of new pandemics will always be with us and the new research center will enhance the world’s preparedness and our speed of reaction the next time we face such a challenge.”
More from Science & Enterprise:
- Trial Okayed in Germany for Covid-19 Vaccine
- Moderna Gains $483M for Covid-19 Vaccine
- Five Covid-19 Vaccines in Clinical Trials
- Covid-19 Vaccine in Development, Trial Planned
- Trial to Test Electronic-Aided Covid-19 Vaccine
* * *

 RSS - Posts
RSS - Posts
[…] Oxford, AstraZeneca Partner on Covid-19 Vaccine […]
[…] Oxford, AstraZeneca Partner on Covid-19 Vaccine […]
[…] Oxford, AstraZeneca Partner on Covid-19 Vaccine […]