30 Oct. 2020. The Food and Drug Administration plans to use organ-on-chip models to test mechanisms for preventing Covid-19 infections, and other processes. The company Emulate Inc. in Boston says it signed a cooperative research agreement with FDA to evaluate these organ-on-chip devices for studying the safety, efficacy, and mechanisms of its regulated drugs.
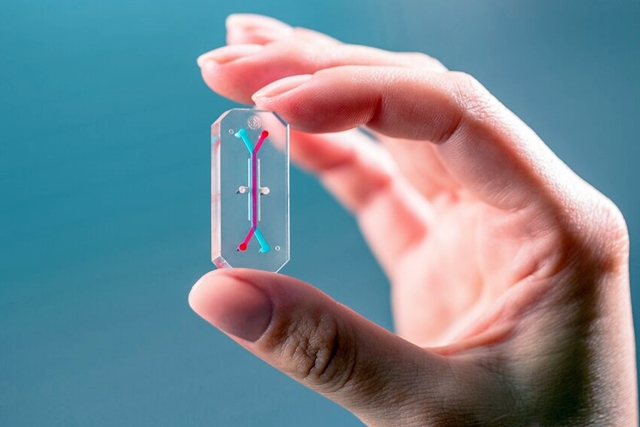
Emulate Inc. is a spin-off from Harvard’s Wyss Institute for Biologically Inspired Engineering that conducts research on the science behind these devices. Organs-on-chips are clear, polymer plastic strips about the size of flash memory sticks, with fine channels lined with human cells and tissues that replicate the functions of organs in the body. They provide a more controlled and predictive way of testing the toxicity of drugs, chemicals, and cosmetics than lab animals, and also have potential for testing personalized treatments for diseases.
The agency aims to use Emulate’s lung chip to demonstrate the mechanisms for providing immune system protection against the SARS-CoV-2 virus responsible for Covid-19 infections. The company says this chip recreates the microenvironment in human lungs, showing mechanical strains and fluid flow. The study team will infect lung chips with various SARS-CoV-2 strains to explore infection susceptibility, and examine immune responses from human blood plasma samples with neutralizing antibodies. The team also plans to investigate the potential for antibody dependent enhancement, where an antibody binding to its target can facilitate further infections instead of invoking an immune response.
FDA plans as well to use Emulate Inc. chips to investigate other disease and therapy processes:
– Brain chip, to model Alzheimer’s disease, where adult stem cells are transformed into different types of brain cells to better understand characteristics of Alzheimer’s disease, and to test for beneficial or toxic effects of treatments
– Intestine chip, to study microbiome physiology and effects of food residues on microbial communities in the gut, particularly development of antimicrobial bacteria
– Liver chip, with cells from various donors to test for toxicity responses, and identify biomarkers in the liver that indicate susceptibility to liver toxicity, a continuing issue with some drugs
The FDA deal with Emulate Inc. is called a Cooperative Research and Development Agreement, or Crada, that allows for transfer of government-owned intellectual property or know-how to a private company or institution for mutual benefit. Crada agreements are used to define collaborative projects, often without funds payments from one party to another. In 2017, FDA signed a Crada with Emulate to evaluate the company’s organ chips for toxicity testing.
“We are enthusiastic to collaborate with essential regulatory partners like the FDA,” says Lorna Ewart, Emulate’s executive vice president for scientific liaison in a company statement, “to apply our unique organ-on-chips platform to advance the understanding of the SARS-CoV-2 virus and to do our part in bringing safe and effective Covid-19 vaccines and treatments to the world.”
More from Science & Enterprise:
- Software Designed to Model, Visualize Brain Circuits
- NIH Expands Covid-19 Test Technology Challenge
- Partnership to Speed Covid-19 Clinical Trials
- NIH Opens Covid-19 Diagnostic Test Challenge
- Digital Health Research Platform Set for Covid-19
* * *

 RSS - Posts
RSS - Posts
[…] FDA to Use Lung Chip Model for Covid-19 Testing […]
[…] FDA to Use Lung Chip Model for Covid-19 Testing […]