Charité – Universitätsmedizin Berlin in Germany now offers for selected patients a nanomedicine method for the treatment of recurrent brain tumors. The science underlying the nanotechnology-based cancer therapy was developed by researchers at Charité, which is being marketed by MagForce Nanotechnologies AG, a Charité spin off company.
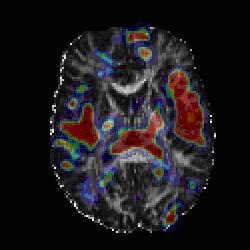
The principle behind the therapy is the use of nanoscale particles (one nanometer equals one billionth of a meter) containing iron oxide, which are injected into a brain tumor in a procedure similar to a biopsy. The treatment is carried out in a magnetic field applicator, a machine that produces an alternating magnetic field, yet is safe for humans.
Through this high-frequency magnetic field, the nanoparticles begin to oscillate and heat is produced from inside the tumor tissue. Depending on the temperature reached and length of treatment, the tumor cells are either directly destroyed or sensitized for the accompanying chemotherapy or radiation. Charité says the therapy has the potential to improve the survival for patients with recurrent glioblastoma, a malignant type of brain tumor.
A clinical trial supporting the therapy’s European approval was conducted in close collaboration with Charité. The medical center says, however, that social insurance companies generally do not reimburse patients for this treatment option, thus most individuals will need to cover the costs of the therapy.
Read more: Research Institutes Collaborate on Cancer Nanomedicine
* * *

 RSS - Posts
RSS - Posts
Nanotechnology has done a great deal in the artificial pacemaker for cardiovascular diseases and its role could be vital in treating cancer! Thanks for the share.
best Cancer Care Hospital in India
[…] Medical Center Commercializes Nanotech Cancer Treatment […]
Which hospitals in Germany or the UK offer this treatmeny? Thanks
Thank you Nassir for your question. I would suggest you contact MagForce Nanotechnologies that is commercializing the treatments. The company’s contact information is found at http://www.magforce.de/kontakt.html . Best regards and thank you for reading Science Business. – AK