St. Jude Medical in St. Paul, Minnesota says it received the European CE Mark approval for its implanted neurostimulation device for patients with chronic migraines. The CE marking (an acronym for the French “Conformite Europeenne”) certifies that a product has met EU health, safety, and environmental requirements for consumer safety.
The company’s Genesis system for peripheral nerve stimulation (PNS) of the occipital nerves is designed for the management of the pain and disability associated with intractable chronic migraine. This type of migraine is defined as headache lasting at least four hours per day for 15 or more days per month, causing at least moderate disability, and not responding to three or more preventive drugs.
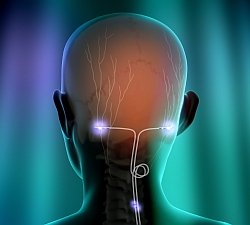
PNS therapy (illustrated at right) for this condition involves the delivery of mild electrical pulses to the occipital nerves that are located just beneath the skin at the back of the head. A small electrical lead or leads are placed under the skin and connected to the neurostimulator which produces the pulses of stimulation.
St. Jude says the CE Mark approval was based on the results of a randomized, double-blind, controlled clinical trial that collected data from 157 patients. Patients enrolled in the study suffered from headache, on average, 26 days per month.
The findings indicate that at 12 weeks, participants in the active group had 41 percent lower overall disability compared to a 13 percent reduction in the control group, based on the Migraine Disability Assessment (MIDAS) questionnaire. Also at 12 weeks, patients who received stimulation had 36 percent fewer headache days compared to the control group which reported a 25 percent decrease.
Of the patients who used the PNS device for a year, about two-thirds (65 to 68 percent) each reported excellent or good pain relief, said that their quality of life had improved, and were satisfied or very satisfied with the results of their procedure.
Read More: Study Explains How Tinted Lenses Offer Relief from Migraines
* * *

 RSS - Posts
RSS - Posts
[…] CE Mark Approved for Migraine Implant Therapy […]
[…] year, St. Jude received CE approval for its Genesis line of neurostimulators, which also delivers peripheral nerve stimulation to the occipital nerves. A […]