Tandem Diabetes Care Inc. in San Diego says the Food and Drug Administration has cleared the company’s insulin pump system for marketing in the U.S. The t:slim Insulin Delivery System, as it’s called, is one of the first insulin pumps to be cleared under the FDA’s new Infusion Pump Improvement Initiative, according to the company.
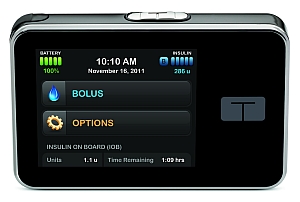
The t:slim is a programmable insulin infusion pump for people with diabetes, age 12 and older who require insulin. The pump’s interface features a color touch screen designed to make diabetes management easier. The device provides accurate insulin delivery from a 300-unit cartridge.
Diabetes, according to the Centers for Disease Control and Prevention, affects 25.8 million people, about 8.3 percent of the U.S. population. It is the leading cause of kidney failure, non-traumatic lower limb amputations, and new cases of blindness among adults in the United States, a major cause of heart disease and stroke, and the seventh leading cause of death in the U.S.
A 2008 white paper by the American Association of Diabetes Educators says insulin pumps make it possible to provide diabetes patients with customized insulin dosages while reducing the risk of severe hypoglycemia. Delivery of insulin through a pump can be more consistent and precise than delivery by syringe or injection pen.
The FDA established its Infusion Pump Improvement Initiative to address what it calls persistent safety problems with infusion pumps of all kinds, including insulin pumps, notably software errors and badly designed or confusing user controls. FDA’s program urges manufacturers of infusion pumps to meet with FDA early in the development process to identify potential issues and voluntarily submit their software for review. FDA also held a workshop for pump manufacturers and developed collaborative research projects to improve pump management software.
Read more: FDA Approves Type 2 Diabetes Treatment
* * *

 RSS - Posts
RSS - Posts
[…] FDA Approves Touch-Screen Insulin Pump System […]