Researchers from Boston University and Harvard medical schools have devised an inexpensive, point-of-care prototype device that tests for flu strains. The team led by BU biomedical engineering professor Catherine Klapperich published its findings last week in the journal PLoS ONE.
The lab test currently considered the most accurate for influenza uses a process called reverse transcription polymerase chain reaction or RT-PCR. The test, which takes about three hours, normally requires expensive bench-top testing machinery and highly trained lab technicians. The process extracts ribonucleic acid (RNA) from nasal specimens, where the RNA is reverse-transcribed into DNA, and the DNA is then replicated into sufficient quantities to be analyzed by an external DNA reader
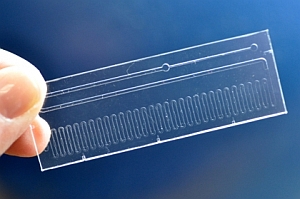
Klapperich’s team incorporated those functions on a single-use microfluidic chip that can be used in a doctor’s office. The device is about the size of a microscope slide, and performs the same functions as RT-PCR equipment. The top part of the chip takes RNA from the specimen sample, the middle section converts the RNA to DNA, and the bottom section heats and replicates the DNA to be analyzed by a separate instrument.
The researchers tested 146 samples for influenza A and B with the microfluidics chip, and then with the lab-based technology. The results show the microfluidics chip returned results that matched the output of the bench-top equipment, and in about the same amount of time.
The team also compared the microfluidic process against other testing technologies: viral culture testing, rapid immunoassays that work like home pregnancy tests, and direct fluorescent antigen (DFA) testing. The results show microfluidics ships are faster than viral culture testing that takes about a week. Rapid immunoassays are fast, but were reliable only 40 percent of the time in detecting flu viruses. DFA testing was more accurate than the chips, but required a more labor-intensive process for staining and interpreting samples with flourescent antibodies.
“Making each chip single-use decreases the possibility of cross-contamination between specimens,” says Klapperich, “and the chip’s small size makes it a good candidate for true point-of-care testing.”
The next stage of the development process say the researchers is to refine the process to produce results in about one hour, with chips that cost half as much to make (five dollars). The team is also exploring ways to develop a lower cost external DNA reader that’s no bigger than a clinical digital thermometer.
Read more: Faster, More Sensitive Flu Diagnostics Developed
* * *

 RSS - Posts
RSS - Posts
[…] Lab-On-A-Chip Device Developed to Test for Flu Viruses […]
[…] Lab-On-A-Chip Device Developed to Test for Flu Viruses […]
[…] Lab-On-A-Chip Device Developed to Test for Flu Viruses […]